What Happens When the World Speeds Up and Our Brains Slow Down?
There's a moment most people over fifty recognize but can't quite name. You are having a meal with friends or family, and the conversation shifts faster than you can track. Or you're merging onto a busy road and the gap between the cars and open lane closes before you get there. Or you are installing a new app on your device or creating a new online account, and by the time you've absorbed step two, they're on step five.
Nothing is wrong with you. Your reasoning is intact. Your words are there. Your judgment is sound. You have more knowledge than ever. But it feels like you are losing a fraction of a second each day.
That something is processing speed — the rate at which your brain takes in information, makes sense of it, and produces a response. And understanding what happens when it changes is one of the most important things you can do for yourself or someone you love as you age.
Processing Speed
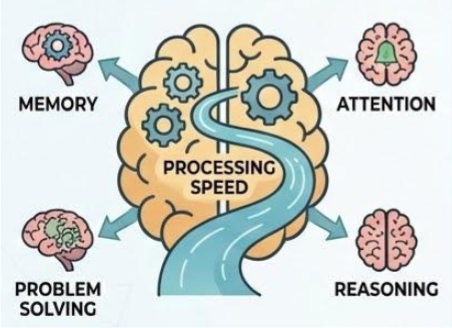
Processing speed isn't intelligence. It isn't memory. It isn't wisdom. It's the tempo of your thinking — how quickly your brain can manage and use information.
Think of it this way: if cognition is an orchestra, processing speed is the conductor's tempo. The musicians (your reasoning, your knowledge, your judgment) may be as talented as ever. But when the tempo slows, the whole performance changes. Some passages that require precise coordination start to fall apart. Some musicians can't finish their parts before the next section begins.
In 1996, Timothy Salthouse at Georgia Tech published what became the foundational theory of how processing speed relates to cognitive aging. His processing-speed theory proposed two mechanisms that explain why slower processing degrades cognitive performance, even when the underlying abilities are preserved (Salthouse, 1996, Psychological Review).
The first is the limited time mechanism. When processing is slow, you simply can't complete all the mental operations you need to within a given window. The conversation moves on. The traffic gap closes. The app tutorial leaves you behind. It's not that you can't understand — it's that the world doesn't wait.
The second is the simultaneity mechanism, and this one is more subtle. Many cognitive tasks require you to hold several pieces of information in mind at the same time — to see the relationships between them, to integrate them into a decision. But information doesn't stay active in your mind forever. It fades. And if your processing is slow, the early pieces of information may have already degraded by the time you've finished working through the later ones. You're trying to assemble a puzzle, but the first pieces keep dissolving before you can place the last ones.
Salthouse's insight was that these two mechanisms together can explain an enormous proportion of age-related cognitive change. In his studies, controlling statistically for processing speed eliminated roughly 75% or more of the age-related variance in measures of memory, reasoning, and spatial ability. That's a staggering number. It means that much of what we call "cognitive aging" isn't really about losing the ability to think — it's about losing the speed to think in a world that demands rapid coordination of information.
When Does Processing Speed Change?
Here's the part that surprises most people: processing speed starts declining in your late twenties.
That's not a typo. The research is remarkably consistent on this point. Fluid cognitive abilities — the ones that require you to process novel information, solve new problems, and respond quickly — begin a gradual, largely linear decline starting around age 25-30 and continuing throughout the lifespan. Change is imperceptible year to year but unmistakable decade to decade.
By contrast, crystallized abilities — your vocabulary, your accumulated knowledge, your expertise — continue to grow well into your sixties and seventies. This is why a sixty-year-old attorney may be a far better strategist than her thirty-year-old associate, even though the associate can process a new brief faster. The older attorney has seen more patterns, built deeper frameworks, and developed an intuitive sense for what matters. Her processing speed has slowed, but her wisdom — in the truest cognitive sense — has grown.
This divergence between crystallized and fluid abilities is one of the most important facts about cognitive aging. It means that as you age, you're not simply declining. You're changing shape. Some dimensions of your mind are shrinking while others are expanding. The question isn't whether you're getting "better" or "worse" at thinking. The question is: how do you reorganize your life to play to your strengths?
There Is More Than One Decline Curve
The story of cognitive aging is richer than a single curve heading downward.
Laura Germine is a cognitive scientist who co-created TestMyBrain.org, one of the first online cognitive testing platforms. Through that platform and others, Germine and her colleagues have collected cognitive data from millions of volunteers — an unprecedented scale that allowed them to map when different mental abilities actually peak across the entire lifespan.
Processing speed peaks early, as Salthouse predicted — around age 18 or 19. Short-term memory improves until about 25 and begins declining around 35. So far, consistent with the standard picture. But then things get interesting. Working memory climbs into the late 20s and early 30s and declines only slowly. Social cognition — the ability to read other people's emotions — peaks in the 40s and doesn't start to significantly decline until after 60. And vocabulary? It just keeps improving, all the way into the late 60s and early 70s.
In other words, there is no single peak for cognitive ability. Different capacities are on different timelines, and some are still developing well into midlife. Your brain at 55 or 65 or 75 or 85 isn't just a slower version of your brain at 25. It's a fundamentally different instrument — worse at some things, better at others, and still changing in ways that matter enormously for real-world functioning.
The World Got Faster Too
Here's what makes the processing speed story so much more urgent than it was when Salthouse published his theory in 1996: the world has changed at least as much as our brains have.
In 1996, most people got their news from a morning paper and an evening broadcast. Email was a novelty. The internet was a curiosity. There were no smartphones, no social media feeds, no push notifications, no algorithmic content streams designed to capture and hold your attention for as long as possible.
Today, the average person is inundated with messages throughout the day. Our phones buzz with notifications. Our inboxes fill faster than we can empty them. Medical information arrives not from a single trusted physician but from a firehose of Google results, patient portals, wellness apps, and well-meaning relatives forwarding articles. Financial information moves in real-time tickers. The news cycle runs 24 hours. Stores don’t close on Sundays.
This means that the challenge of cognitive aging isn't just that our brains are slowing down. It's that our brains are slowing down while the world is speeding up. These two curves — one biological, one technological and cultural — are moving in opposite directions, and the gap between them is widening every year.
And it's not just processing speed, our attention — the gateway through which all information must pass before it can be processed at all — is being reshaped by the world we've built.
Attention in an Age of Distraction
Attention isn't a single thing. Cognitive scientists distinguish between several forms: sustained attention (maintaining focus over time), selective attention (filtering relevant from irrelevant information), divided attention (managing multiple streams of information simultaneously), and attentional switching (shifting focus between tasks).
All of these are affected by aging, but the modern information environment puts particular strain on selective and divided attention — the very capacities that help you sort signal from noise and manage competing demands.
Consider what happens when you sit down to research a Medicare supplemental plan online. You need to compare coverage options across multiple providers, each with different terminology. Pop-up ads compete for your visual attention. Sponsored content is designed to look like legitimate information. Your email pings. A news alert flashes across your screen. Even a young person with peak processing speed would find this challenging. For someone whose processing speed has slowed and whose attentional filtering has become less efficient, it can be genuinely overwhelming.
The result is a kind of cognitive mismatch that didn't exist a generation ago. Our grandparents experienced age-related processing speed decline in a world that moved at the speed of handwritten letters, face-to-face conversations, and decisions that could unfold over weeks. We're experiencing it in a world that moves at the speed of fiber optics and expects responses in minutes.
When the information environment outpaces your ability to process it, you don't just miss things — you become dependent on others to filter, interpret, and act on information for you.
When Disease Accelerates the Clock
Normal aging produces a gradual, manageable slowing. But several diseases of aging can dramatically accelerate the process, creating challenges that require more deliberate adaptation.
Alzheimer's disease is the most well-known. While Alzheimer's is most associated with memory loss in the public imagination, slowed processing speed is one of the earliest and most consistent findings, often detectable years before a clinical diagnosis. In the preclinical window — what I think of as the "hidden window" — processing speed deficits begin to interact with the disease's effects on memory and executive function, creating a cascade that progressively undermines the person's ability to manage complex decisions.
Cerebrovascular disease — the accumulation of small-vessel damage in the brain from conditions like hypertension, diabetes, and atherosclerosis — may be an even more common driver of processing speed decline. White matter lesions, which show up as bright spots on brain MRI, disrupt the connections between brain regions, literally slowing the transmission of neural signals. This is one reason why managing cardiovascular risk factors (blood pressure, blood sugar, cholesterol) is one of the most evidence-based things you can do to protect your processing speed.
Parkinson's disease, multiple sclerosis, traumatic brain injury, and major depression all carry significant processing speed costs. In each case, the mechanisms may differ — dopamine depletion in Parkinson's, demyelination in MS, diffuse axonal injury in TBI, altered neural network efficiency in depression — but the experience is similar: the world feels like it's moving too fast.
The World Changes When The Clock Slows
When processing speed slows significantly — whether from normal aging, disease, or both — and the information environment continues to accelerate, the world doesn't just feel faster. It feels different. The change reshapes your daily experience in ways that are hard to articulate but impossible to ignore.
Conversations become harder to follow. Not because you don't understand the words, but because by the time you've fully processed one point, the speaker has moved on to the next. Group conversations are especially demanding because they require rapid tracking of multiple speakers, shifting topics, and social cues. Many people with slowed processing speed start to withdraw from social situations because the cognitive load of keeping up is exhausting.
Driving becomes riskier. Driving is one of the most processing-speed-dependent activities in daily life. It requires continuous integration of visual information from multiple sources, rapid decision-making, and split-second motor responses. Research using the Useful Field of View (UFOV) paradigm has consistently shown that slower processing speed predicts higher crash risk in older adults. This doesn't mean every older adult is an unsafe driver. It means that when processing speed drops below a certain threshold, the margins of safety narrow in ways that matter.
Financial decisions become vulnerable. Financial decisions — especially the complex, multi-step ones involving investments, estate planning, and major purchases — require exactly the kind of simultaneous information integration that Salthouse's simultaneity mechanism describes. You need to hold the terms of the deal in mind while comparing them to alternatives, while assessing risk, while considering tax implications. When processing speed slows, you may still understand each piece individually, but the ability to hold all the pieces together at once declines. This is when people become vulnerable to poor decisions, scams, and undue influence — not because they've lost their judgment, but because they can't deploy their judgment fast enough to catch the problems.
Technology becomes a barrier. Modern technology is designed by and for fast processors. Interfaces change rapidly, notifications demand immediate attention, and the default assumption is that users can keep up with multi-step, time-sensitive interactions. For someone with slowed processing speed, even routine tasks like online banking, telehealth appointments, or navigating a new phone update can become sources of frustration and avoidance.
Medical decision-making gets harder. Imagine sitting in a doctor's office, receiving a new diagnosis. The physician is explaining treatment options, each with its own risk-benefit profile. You're trying to process the diagnosis itself while simultaneously weighing the options. With slowed processing speed, you may leave the appointment feeling like you understood very little — not because the information was too complex for you, but because it was delivered too fast for your current processing capacity.
When Slower Can Be Better
People who process more slowly tend to be more deliberate. They're less likely to jump to conclusions. They're more likely to weigh evidence carefully before committing to a decision. In situations that reward caution and thoroughness over speed — and many of life's most important situations do — slower processing can actually be an asset.
Research on decision-making across the lifespan supports this. Older adults tend to rely more on gist-based reasoning, focusing on the essential meaning of information rather than getting lost in the details. They're often better at filtering out irrelevant information and zeroing in on what matters. This isn't a deficit — it's an adaptation, and in many contexts, it produces better outcomes.
The problem isn't that slower processing is inherently bad. The problem is that the world isn't designed for it. When you can restructure the environment to match the processor — giving yourself more time, reducing distractions, breaking complex decisions into smaller steps — the preserved reasoning and accumulated wisdom of aging can shine.
What the Evidence Says About Interventions
If processing speed is so central to cognitive aging, can anything be done about it? The answer, increasingly, is yes — with some important caveats.
The strongest evidence comes from the ACTIVE trial (Advanced Cognitive Training for Independent and Vital Elderly), the largest and longest randomized controlled trial of cognitive training in older adults. In a remarkable 20-year follow-up published just this month in Alzheimer's & Dementia: Translational Research and Clinical Interventions (Coe et al., 2026), researchers found that participants who completed speed-of-processing training — about five to six weeks of adaptive computer-based exercises — plus booster sessions one to three years later, had a 25% lower rate of dementia diagnosis over the next two decades compared to the control group.
This is extraordinary for several reasons. First, the intervention was modest: roughly ten sessions of 60-75 minutes each, followed by a few booster sessions. Second, neither memory training nor reasoning training produced the same effect — only speed training did. Third, the effect lasted twenty years. As Marilyn Albert, the corresponding author and director of the Johns Hopkins Alzheimer's Disease Research Center, noted, even small delays in the onset of dementia could have an enormous public health impact.
Why speed training and not the others? The researchers point to a key difference: speed training is adaptive (it gets harder as you improve) and it drives implicit learning — the kind of unconscious skill-building that resembles developing a reflex. Memory and reasoning training, by contrast, taught explicit strategies that everyone in the group learned the same way. Implicit learning engages different brain networks and may produce more durable neural changes.
Physical exercise is the other intervention with strong evidence for protecting processing speed. Aerobic exercise in particular has been linked to preserved white matter integrity, improved cerebrovascular health, and better processing speed performance in both cross-sectional and longitudinal studies. The mechanisms are probably multiple: exercise improves blood flow to the brain, reduces inflammation, stimulates the release of brain-derived neurotrophic factor (BDNF), and helps manage the cardiovascular risk factors that contribute to small-vessel disease.
Cardiovascular risk management — controlling hypertension, diabetes, and hyperlipidemia — protects the brain's white matter infrastructure and may be the single most impactful thing most people can do to preserve processing speed. The SPRINT-MIND trial showed that intensive blood pressure control (targeting systolic pressure below 120 mmHg) reduced the risk of mild cognitive impairment compared to standard treatment.
During sleep, the brain clears metabolic waste products, consolidates memories, and restores neural function. Chronic sleep deprivation and untreated sleep apnea are both associated with reduced processing speed and accelerated cognitive aging.
Adapting Your Life to a Changing Brain
The interventions above are about slowing the decline. But equally important — and often more immediately practical — is adapting your life to work with your changing brain rather than against it.
Here's what that looks like in practice:
Give yourself more time. This sounds obvious, but it requires a genuine restructuring of how you approach tasks. If you used to review financial documents the night before a meeting, start reviewing them three days before. If you used to make medical decisions in the appointment, start requesting information in advance so you can process it at your own pace.
Curate your information environment. This may be the most underappreciated adaptation available to you. Unsubscribe from email lists that create noise. Turn off non-essential notifications on your phone. Designate specific times for checking news rather than letting it wash over you all day. When you need to research something important — a medical decision, a financial product, a legal question — don't start with an open-ended Google search. Start by asking a trusted professional or knowledgeable person to point you to the two or three sources that actually matter. Your goal isn't to see everything. Your goal is to see the right things with enough time and focus to process them well.
Protect your attention like you protect your money. Every app, every platform, every notification is asking for a piece of your attention — and attention is a finite cognitive resource that becomes more precious as processing speed declines. Be as deliberate about where you spend your attention as you are about where you spend your dollars.
Reduce the need for simultaneous processing. Break complex decisions into sequential steps. Write things down. Use checklists. These aren't signs of weakness — they're intelligent adaptations to a known change in your cognitive profile.
Protect high-stakes decisions. Identify the areas of your life where processing speed matters most — finances, medical decisions, legal matters, driving — and build in extra safeguards. Get a second opinion before major financial moves. Bring a trusted person to important medical appointments. Be honest with yourself about your driving, especially at night, in new places, and in heavy traffic.
Stay socially engaged, but on your terms. Social isolation is a major risk factor for cognitive decline, but overwhelming social situations can drive withdrawal. Find social contexts that work for you — smaller groups, quieter settings, one-on-one conversations at your pace.
Keep learning, but differently. The ACTIVE trial suggests that the right kind of cognitive challenge can have lasting benefits. You don't have to "use it or lose it" in a generic sense — but targeted engagement with activities that challenge your processing speed (like the BrainHQ Double Decision exercises used in the ACTIVE trial) may offer genuine protection. This could be paired with physical exercise for a potentially synergistic effect.
The Science Is Catching Up
There's a hopeful thread running through all of this that's worth making explicit.
When Salthouse published his processing-speed theory in 1996, he gave us a powerful framework for understanding what was happening in the aging brain and why. But the tools available to act on that understanding were limited. Cognitive assessment was something that happened in a clinic, administered by a specialist, scored by hand, and typically deployed only after someone was already showing obvious problems. The idea of detecting subtle processing speed changes before symptoms appeared was science fiction.
When Germine launched TestMyBrain in 2008, the paradigm started to shift. For the first time, rigorous cognitive testing could happen anywhere, on any device, with anyone who had an internet connection. The data from millions of volunteers proved that digital assessment works. It showed that you didn't have to bring people into a laboratory to measure their brain function with scientific rigor.
What does this mean for you? The ability to detect subtle processing speed and cognitive changes during that 7-10 year window before a dementia diagnosis will become widely accessible, scientifically validated, and free. The tools that require a visit to a neuropsychologist's office could be available on your personal device.
This won't replace the need for expert guidance in navigating what comes next. A test that tells you your processing speed is declining doesn't tell you how to restructure your financial decision-making, or when to have the conversation about driving, or how to protect your autonomy while accepting appropriate support. But it could fundamentally change when those conversations begin — shifting them from reactive crisis management to proactive planning. And that shift, from late to early, from crisis to preparation, may be the most consequential change in how we approach cognitive aging in a generation.
The Bottom Line
Two things are happening at once. Your brain is gradually slowing down — a process as natural as your hair turning gray. And the world around you is speeding up, demanding faster responses to more information through more channels than at any point in human history. The collision of these two trends is the defining cognitive challenge of aging in the 21st century.
But the science is catching up. From Salthouse's discovery that processing speed is the central driver of cognitive aging, to Germine's revelation that different cognitive abilities peak on their own timelines, to building the tools to detect the earliest changes before symptoms appear. We are closer than we have ever been to understanding what's happening in the aging brain and intervening at the right time.
The evidence-based steps you can take today are clear: speed-of-processing training with booster sessions, regular aerobic exercise, cardiovascular risk management, and adequate sleep. And equally important, you can adapt your life to work with your changing brain — by giving yourself more time, curating your information environment, protecting your attention, reducing the need for simultaneous processing, safeguarding high-stakes decisions, and building the external structures that let your preserved reasoning and accumulated wisdom do their best work.
The world will keep speeding up. Your brain will keep its own pace. But with the right understanding and the right adaptations, you can continue to move through it with competence, dignity, and confidence. The music hasn't stopped. The tempo has changed. And you get to choose how you play it.
References
Coe, N. B., et al. (2026). Impact of cognitive training on claims-based diagnosed dementia over 20 years: Evidence from the ACTIVE study. Alzheimer's & Dementia: Translational Research and Clinical Interventions, 12(1). DOI: 10.1002/trc2.70197
Hartshorne, J. K. & Germine, L. T. (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the lifespan. Psychological Science, 26(4), 433-443.
Salthouse, T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403-428.
Williamson, J. D., et al. (2019). Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial (SPRINT-MIND). JAMA, 321(6), 553-561.
The Science of Seeing Sooner: How Cutting-Edge Research Is Changing What's Possible for Families
Earlier this week, Georgetown University's Center for Security and Emerging Technology published an analysis of how prize competitions can accelerate AI-driven scientific discovery. They were examining which competitions best exemplify how we can use innovation challenges to advance science — and they chose the PREPARE Challenge as their lead example.
PREPARE — Pioneering Research for Early Prediction of Alzheimer's and Related Dementias — was a competition to improve detection of cognitive decline before a clinical diagnosis, by bringing together data scientists, clinicians, and AI researchers to build better prediction tools.
I led that challenge. It was one of the most rewarding experiences of my career — seeing a concept move from whiteboard to execution to a launchpad for translation into clinical practice. It came in on the tailwinds of a revolution that is shaking up how we treat diseases like Alzheimer’s disease.
A Revolution in Early Detection
For decades, the tools available for detecting Alzheimer's disease and related dementias were blunt instruments. By the time a diagnosis arrived, years of gradual change had already passed — years when families might have planned differently, acted sooner, or simply understood what they were seeing.
That reality is changing fast, across multiple scientific fronts at once.
The blood is talking. A new generation of blood tests can now detect the biological signatures of Alzheimer's disease — the protein buildups in the brain that were previously visible only through expensive brain scans or spinal taps. One of these tests received FDA approval in 2025. A simple blood draw can now provide information that once required a $5,000 imaging procedure. These tests aren't perfect screening tools yet, but they're transforming how clinicians evaluate cognitive concerns, especially when combined with other information.
Digital tools are catching what traditional tests miss. Researchers have developed computerized cognitive assessments that measure not just whether you got the answer right, but how you got there — the hesitations, the timing, the subtle shifts in processing speed that a paper-and-pencil test can't capture. Some of these tools can identify risk years before traditional screening methods raise a flag.
Voice and language patterns carry hidden signals. One of the most striking findings from recent research — including work that emerged through PREPARE — is that changes in speech and language can contain early markers of cognitive change. The way someone organizes a sentence, pauses between thoughts, or retrieves words can shift in detectable ways well before anyone in the room notices.
AI is connecting the dots. Machine learning algorithms can now integrate information across these different streams — blood markers, cognitive performance, speech patterns, medical history, even social and environmental factors — to build prediction models that outperform any single measure alone. PREPARE was designed to accelerate exactly this kind of work, and the results confirmed that combining diverse data sources yields meaningfully better early detection.
What the Science Can Do vs. What Families Actually Receive
Here's the gap that motivated to start Treasure Coast Cognition.
This revolution in early detection is real. The science works. But almost none of it reaches families during the window when it matters most.
During this window, if you bring cognitive concerns to your doctor, you'll likely hear some version of: "It's probably normal aging. Let's keep an eye on it." That's not negligence — it's a system that isn't designed to act on early, ambiguous signals.
Meanwhile, the science now exists to characterize those early changes with far greater precision. Blood biomarkers can clarify biological risk. Sensitive cognitive assessments can establish baselines and track trajectories over time. Integrative analysis can distinguish between normal age-related variation and something that warrants closer attention and proactive planning.
The problem isn't the science. The problem is that no one is translating it for families in a way that leads to action.
Why I Built Treasure Coast Cognition
The neuroscience of cognitive aging, the early behavioral and biological markers of decline, the gap between research capability and clinical reality is the reason for Treasure Coast Cognition.
The clearest thing I learned was this: the families who navigate cognitive change best are the ones who start paying attention early — not after a crisis, not after a diagnosis, but during the window when understanding and planning are still fully available.
That's what Cognitive Stewardship is. It's the practice of proactively monitoring cognitive health, interpreting the signals that matter, and building a plan that protects independence, finances, and family relationships — grounded in the best available science.
Treasure Coast Cognition exists to be the bridge between what cutting-edge research can now detect and what your family actually needs to know and do. We take the same science that Georgetown just highlighted as a model for innovation, and we make it personal, ongoing, and actionable.
What This Means for You
If you're watching a parent, spouse, or loved one and wondering whether what you're seeing is "just aging" or something more — that uncertainty itself is a signal worth taking seriously.
Not because it necessarily means something is wrong. But because the science now exists to give you a clearer answer than "wait and see." And because the decisions that matter most — about legal authority, financial oversight, care preferences, living arrangements — are far better made with time and clarity than under pressure.
The revolution in early detection isn't coming. It's here. The question is whether your family has access to someone who can translate it into guidance that actually matters for your life.
That's the work we do.
"The Hidden Window" at the Harvard Club of Vero Beach
I'm honored to present "The Hidden Window" at the Harvard Club of Vero Beach next month. This talk explores one of the most important—and most overlooked—opportunities families face when a loved one's cognition begins to change.
Why This Matters
Most families wait for a dementia diagnosis before taking action. But by then, the window for proactive planning has largely closed.
Research consistently shows that cognitive and financial decision-making changes begin 7-10 years before a formal diagnosis. This is precisely when families can shape outcomes best—yet most don't realize they're in this critical window.
What We'll Cover
The presentation addresses four key areas:
The Hidden Window — Understanding when cognitive decline actually begins versus when it gets diagnosed, and why this gap matters for families.
Advances in Detection — New biomarkers, blood-based testing, and digital tools that are transforming early identification.
Proactive Stewardship — What it looks like to navigate cognitive change with a strategic plan rather than crisis management.
Practical First Steps — Concrete guidance for families who are noticing changes and wondering what to do next.
The Core Message
If you're noticing changes in a parent, spouse, or loved one—or if you're a professional advisor whose clients are asking difficult questions about cognitive capacity—the window for meaningful action is open now. Waiting has real costs: lost planning opportunities, financial vulnerability, family conflict, and missed treatment windows.
The good news? Proactive planning during this hidden window can prevent most of these outcomes while preserving autonomy and family harmony.
Join Us
This presentation is open to Harvard Club members and guests. If you're interested in attending, register through the Harvard Club of Vero Beach website. If you are not a Harvard affiliate, you are my guest!
Is Cognitive Stewardship Even About Cognition?
I'm a neuropsychologist. I spent twenty years studying how brains change over time. My practice is called Treasure Coast Cognition. The framework I've built is called Cognitive Stewardship.
So it's a fair question when I say: this work isn't really about cognition.
Or more precisely—cognition isn't the point. It's the entryway.
What People Think
People hear "cognitive" and make assumptions. They picture memory tests. Brain scans. A clinical report that says whether something is wrong.
Yes, comprehensive neuropsychological assessment is part of the product. It matters.
But families don't want video game scores. They come because they're watching someone they love change—and they don't know what to do about it.
The question that keeps us up at night is less "what's the diagnosis?" and more "what happens now?"
The Window
Here's what I've learned:
There's a window. It opens when someone first notices that something is different—in themselves or in someone they love. A moment of confusion that lingers. A decision that doesn't quite make sense. A capability that was there last year and isn't there now.
The window closes when clarity is gone—when the person at the center can no longer participate meaningfully in decisions about their own life.
Between those two points, everything important happens. Or it doesn't.
Families update legal documents or they don't. They have hard conversations about money, safety, and care—or they avoid them. They make proactive choices together, or they wait until crisis forces reactive ones.
The window is where agency lives.
Where Does Cognition Fit?
Cognition helps us predict when the window opens and when it closes.
Subtle cognitive changes are often the first signal that the window has opened—before a diagnosis, before a crisis, before anyone uses words like "dementia" or "decline." The changes might be barely noticeable to anyone outside the family. But they're there. And they mean something.
Cognitive assessment can tell you where someone is in that window. Not just "impaired or not impaired," but a much richer picture: Which capacities are preserved? Which are vulnerable? How much runway remains for complex decision-making? What's likely to change next, and when?
That information matters—not because a score is valuable in itself, but because it tells you how much time you have to do the work that actually matters.
The Work That Actually Matters
The work isn't cognition. The work is what you do with the window while it's open. It’s the lantern we take down the path as we navigate the decisions that matter most.
Structuring decisions that need to be made. Making decisions differently as things change over time. Creating permission for conversations that feel impossible. Helping families align when they see things differently. Building a plan that accounts for what's coming—not just what's here.
The questions at the center of this work aren't clinical. They're human:
Where does this end up?
Who decides when I can't?
How do I maintain dignity when things I could do become things I can't?
What can I settle now, while I still have clarity?
These questions belong to anyone watching their own capacity change—for any reason, at any pace. Cognition is one path into that territory. It's not the only one.
What Cognitive Stewardship Actually Provides
Structured partnership sounds abstract until you see what it replaces: fragmented information, undetected cognitive change disconnecting decision-making from behavior, behavior disconnected from what’s happening in the brain, and decisions made in a confused state, in a strange land, under pressure without a clear picture.
Here's what families get:
Clarity about where you stand. Not a screening test or a quick office visit—a comprehensive evaluation that maps cognitive strengths and vulnerabilities in detail. You stop guessing and start knowing.
A realistic timeline. Assessment tells you how much runway you have for complex decisions. That changes everything about what you prioritize and when.
Permission to have the hard conversations. An expert third party creates space for families to discuss what feels undiscussable. When I facilitate, concerns can surface without damaging relationships.
Decision guidance when stakes are high. Should we sell the house? Is it time to transfer financial management? Can Dad still drive safely? These aren't medical questions—but they have cognitive dimensions. You get expert perspective at the moments that matter.
A partner who tracks change over time. One evaluation is a snapshot. Longitudinal monitoring shows trajectory—so you're never surprised by what comes next. We align decisions and what matters most to where you are and where you will go.
Coordination with your existing team. Your estate attorney, financial advisor, and physicians need cognitive perspective to do their jobs well. We provide that.
What Changes
Families who engage in Cognitive Stewardship move from watching and worrying to acting with clarity. They stop waiting for crisis to force decisions. They use the window while it's open.
That's what you're purchasing: not a test, but a partnership through the years that matter most.
How Do I Know You Can Deliver This?
Fair question. Anyone can describe a philosophy. Execution is different.
This is the applied version of everything I've done. You'll tell me how it's working, and we'll deliver better results together.
A Letter To My Founding Families One Year Later
Dear Founding Families,
A year ago, I sent you a letter. I told you that you were taking a chance on something that was new, something we would build together. I told you I didn't take that lightly.
I want to tell you what happened.
What I Thought Would Happen
I had a plan. I had assessments and protocols and frameworks. I had ideas about how this would unfold—how the check-ins would work, what the family meetings would look like, when decisions would need to be made.
Some of that was useful. A lot of it had to change.
You taught me it was about us and the real work doesn't fit neatly into protocols. It happens in the phone calls that weren't scheduled, the questions that came up between meetings, the moments when something shifted and we needed to talk. It happens when a daughter calls because she noticed something new and isn't sure if it matters. It happens when a spouse says, for the first time, "I'm scared."
The frameworks helped. But the relationship is what made it work.
What Actually Happened
What would have happened without this year together? That's the hard thing about prevention—we can't prove what didn’t happen. We can't point to the crisis that didn't happen and say, "See? We stopped that."
But this was the journey we both observed.
There were family conversations that had been avoided because we named a framework for understanding what was happening. We watched adult children move from fear and frustration to something closer to clarity. We watched spouses stop carrying the weight alone.
We watched decisions get made at the right time. Not too early, when they would have felt like an overreaction. Not too late, when the options had narrowed. But in that window when there was still room to choose.
Some of our families are in a different place than they were a year ago. The changes are real, and they're hard. But we're moving through them together, with a plan, instead of scrambling to react.
That's not nothing. That's the whole point.
What I Got Wrong
I want to be honest with you about what didn't work the way I expected.
I underestimated how much time the in-between moments would take—and how important they would be. The scheduled check-ins were valuable, but the unscheduled ones often mattered more. I had to adjust how I structure my time to make room for that.
I overestimated how much families would use the documentation tools I created. Turns out, most of you preferred a phone call to a form. So we talked more and documented less formally. The information still got captured—just differently than I'd planned.
You helped me see what actually matters. The model is better now because of what you taught me.
What You Gave Me
You gave me proof that this works. Proof that we can see, feel, know, and take action around. The kind that comes from watching something unfold over time, seeing it works, and learning that it matters.
You gave me stories I'll carry with me. The hard conversations you had. The decisions you made. The grace you showed each other when things got difficult. I've learned more about how families navigate this passage from walking alongside you than from twenty years of academic research.
You gave me the chance to build something with you in my hometown. That's not a small thing. Family is everything. Family is here. You made it real.
And you gave me your trust—before there was any reason to trust, before there was evidence that this would work. That's a gift I won't forget.
What Comes Next
For most of you, the journey isn't over. There are still decisions ahead, still transitions to navigate, still hard conversations to have. I'll be here for those.
For the practice, there are more families now. They found me because you told them about what we built together. Some of them are at the beginning of the path you've been walking. I'm better able to help them because of what I learned from you.
I don't know exactly what the next year holds. But I know what we did together, and I know it's working. You showed me that. Because you did, other families won't have to.
Thank you for trusting me with your family's story. Thank you for teaching me what this work actually looks like. Thank you for being patient while we figured it out.
I'm grateful. More than I can easily say.
What Changes Brain Health vs. What Changes Alzheimer’s
If you've read about Alzheimer's disease, you've probably encountered two contradictory messages.
The first: Do these ten things and you can prevent Alzheimer's.
The second: There's nothing you can do — and the drugs don't really work anyway.
As with most false dichotomies, both are wrong. And the confusion between them causes real harm — leading families toward either false hope or premature resignation, neither of which serves them.
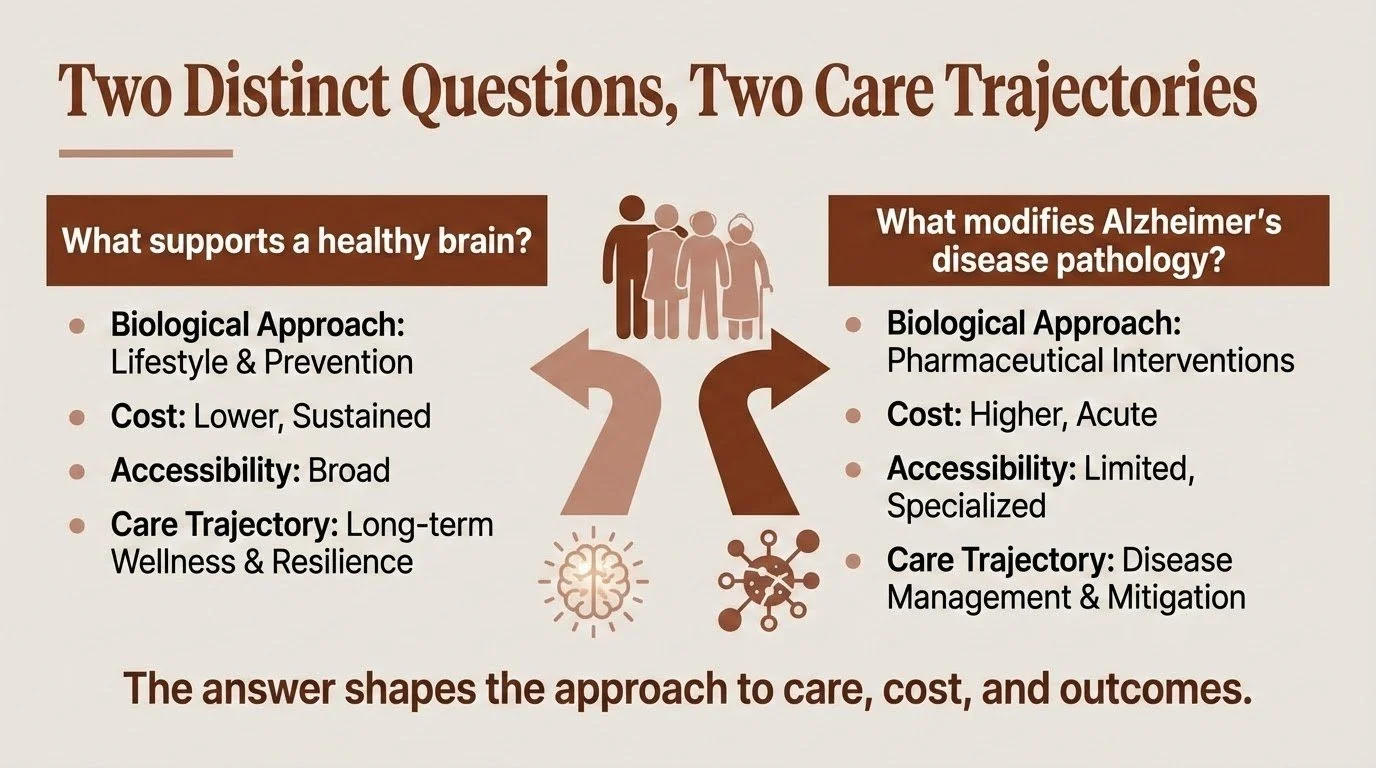
The problem is that most conversations about brain health blur together two fundamentally different questions:
What supports a healthy brain?
and
What modifies Alzheimer's disease pathology?
These aren't the same question. They differ not only in biological approach but also in cost, accessibility, and what they can realistically deliver. Understanding why — and what that means for you and your family — is one of the most important things I can help clarify.
Brain Health: Building Resilience
When researchers talk about lifestyle factors and brain health, they're describing something real and evidence-based. Regular physical exercise, quality sleep, cognitive engagement, social connection, managing vascular risk factors — these work.
At a 2019 National Academies workshop on brain health, I proposed a working definition of resilience that captures what we're actually building: A resilient person has a variety of neurocognitive tools and networks that can be activated in response to challenges, and can adaptively deploy these tools to optimize function when facing environmental and psychological demands. The Collaboratory on Research Definitions for Reserve and Resilience in Cognitive Aging and Dementia has developed consensus definitions distinguishing cognitive reserve, brain maintenance, and brain reserve — with resilience as an overarching framework. Resilience isn't about preventing damage — it's about having options when damage occurs.
The evidence for building resilience is solid. Large trials like FINGER and POINTER have demonstrated improvements in cognitive performance and risk profiles through structured lifestyle interventions. We continue to learn how, including how cognitive reserve builds a more adaptable brain that can compensate longer when damage occurs; vascular health that ensures adequate blood flow; and reduced systemic and neuroinflammatory burden associated with slower decline.
But here's the crucial distinction: these interventions build resilience. They may delay the expression of symptoms. They reduce risk. What they don't do — at least not directly — is stop the molecular machinery of Alzheimer's disease itself.
Importantly, no one "fails" their way into Alzheimer's disease. Genetics and biology matter enormously, even when people do everything right. The lifestyle work is worth doing — but it's not a guarantee, and families shouldn't carry guilt when decline happens despite their best efforts.
And critically, these interventions are accessible. They don't require specialized facilities, insurance approval, or infusion centers. The cost is measured in effort and consistency, not dollars.
Disease Modification: Targeting the Pathology
Alzheimer's disease involves a specific pathological process — the accumulation of amyloid plaques and tau tangles that progressively damage brain tissue. For decades, researchers have worked to develop treatments that target this cascade directly.
The timeline of progress here is worth understanding, because much of what we can now measure and treat has emerged remarkably recently.
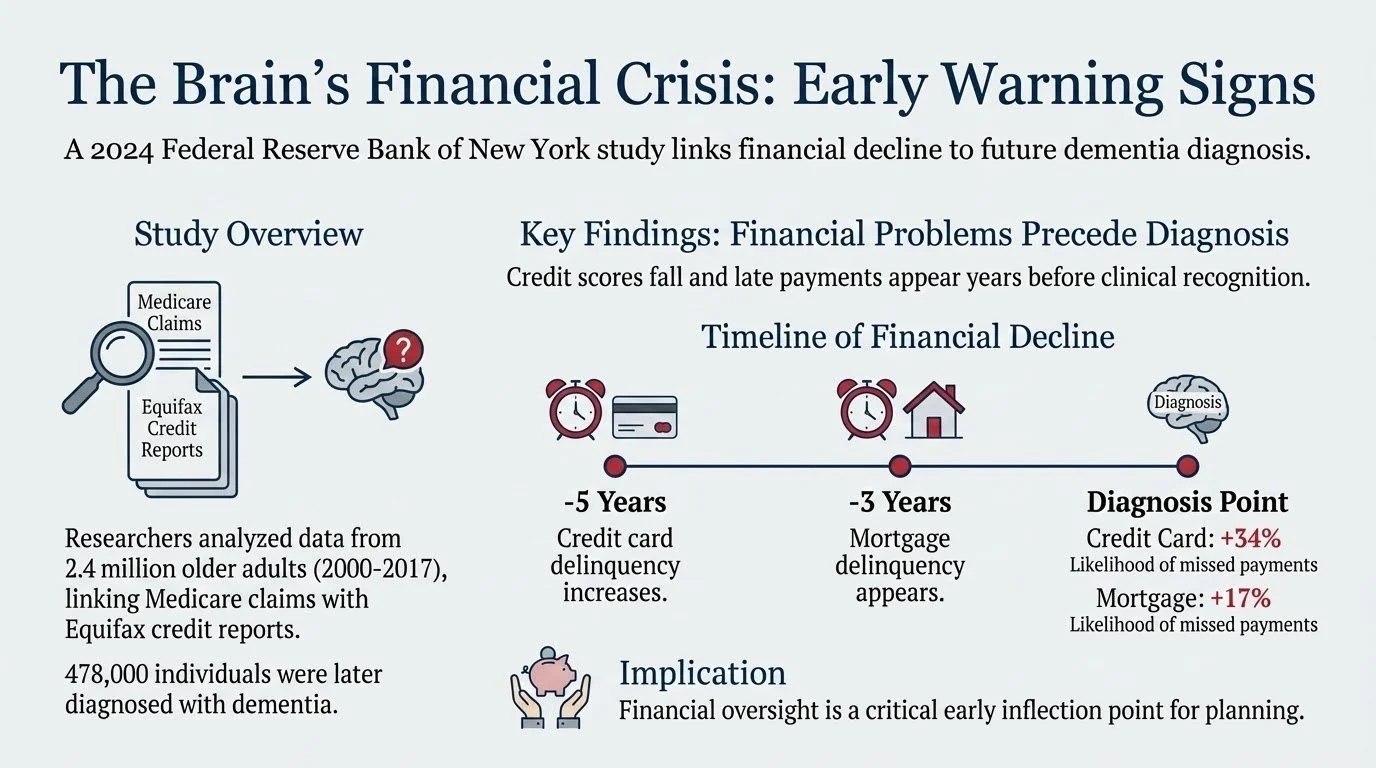
The biomarker revolution: Until the past few years, confirming Alzheimer's pathology required either invasive lumbar punctures for cerebrospinal fluid analysis or expensive PET imaging costing thousands of dollars with limited availability. That's changing rapidly. Blood-based biomarkers — particularly phosphorylated tau at position 217 (p-tau217) — can now detect Alzheimer's pathology with accuracy often approaching PET imaging in research cohorts. Studies show p-tau217 levels begin rising more than 20 years before symptom onset, providing an unprecedented window into what's happening biologically. These tests are becoming available commercially for around $200, though clinical standards for interpretation and follow-up are still evolving.
Detection enables better decisions — even when treatment options are limited. Knowing what you're facing allows for realistic planning, targeted monitoring, and informed choices about emerging therapies.
Disease-modifying therapies: Anti-amyloid antibodies like lecanemab (Leqembi) and donanemab (Kisunla) represent genuine scientific progress. They can reduce amyloid burden in the brain. Clinical trials have shown modest slowing of decline in people with early-stage disease — approximately 25-35% slowing of progression over 18 months.
But "modest" is the operative word. These are not cures. They don't reverse damage already done. Their effects, while statistically meaningful, translate to relatively small differences in day-to-day function over the study periods. Some individuals and families experience noticeable benefit; others experience none. And the treatments come with real risks — brain swelling (ARIA-E) and microbleeds (ARIA-H) requiring careful monitoring with regular MRIs.
A recent episode of the Penn Memory Center's Age of Aging podcast reviewed where things stand after the 2025 Clinical Trials on Alzheimer's Disease (CTAD) conference. The picture is nuanced: anti-amyloid therapies show consistent but modest effects, while hoped-for breakthroughs in other approaches — like GLP-1 medications — have so far disappointed.
Cost and accessibility: This is where disease modification diverges sharply from brain health approaches. Lecanemab costs approximately $26,500 per year; donanemab runs about $32,000. Medicare covers these drugs, but patients face significant out-of-pocket costs — roughly $5,000-6,600 annually after deductibles, plus costs for required monitoring MRIs and infusion visits. The treatments require biweekly (lecanemab) or monthly (donanemab) infusions at specialized centers, genetic testing for APOE status, and ongoing safety monitoring.
One recent improvement: the FDA approved a subcutaneous autoinjector formulation of lecanemab (Leqembi Iqlik) in late 2025 for maintenance dosing. After completing 18 months of IV infusions, patients can now switch to weekly at-home injections — a 15-second self-administered dose that eliminates ongoing infusion center visits. And a supplemental application for subcutaneous initiation treatment — which would allow patients to bypass the IV infusion phase entirely and start treatment at home — was completed in November 2025 and is currently under FDA review.
If approved, this would represent a meaningful shift in accessibility. But for now, many families — particularly those in rural areas or without supplemental insurance — still face significant barriers to starting treatment, regardless of clinical appropriateness.
What may be coming: The next generation of therapies aims to address current limitations. "Brain shuttle" technologies — bispecific antibodies engineered to cross the blood-brain barrier via transferrin receptor binding — show early promise for improving drug delivery while potentially reducing side effects. Roche's trontinemab, which uses this approach, has shown rapid amyloid clearance at lower doses with encouraging early safety data, including very low rates of ARIA-E. In early trials, 91% of participants on the higher dose became amyloid-negative within six months.
These results are early and focused on biomarker outcomes; whether this translates into meaningful clinical benefit remains to be seen. But the direction is promising — better delivery, broader brain distribution, and potentially improved safety profiles.
Why Both Matter — And Why the Distinction Matters More
Here's what I want families to hold:
Do the lifestyle work. It's not nothing. Building cognitive reserve, protecting vascular health, staying engaged — these create real protection. They may give you more good years, more functional capacity, more time. They're accessible to nearly everyone, regardless of insurance status or geography.
And understand that lifestyle isn't a cure. It doesn’t look like exercise or crossword puzzles alone will stop tau from spreading. These are different biological problems requiring different solutions.
Take disease-modifying options seriously — but realistically. For appropriate candidates, anti-amyloid therapies represent a genuine, if modest, advance. But they're expensive, require significant infrastructure, carry real risks, and deliver incremental rather than transformative benefit. They're one tool, not a solution.
The danger comes from conflating these approaches. Families who believe lifestyle alone prevents Alzheimer's may delay necessary planning or feel blindsided when decline happens despite doing "everything right." Families who pin all hope on medications may be devastated by modest results — or may dismiss the meaningful protection that lifestyle factors actually provide.
Neither position serves you.
What This Means for Your Family
People living with Alzheimer's consistently tell us two things matter most: being treated as whole people — not diagnoses — and having time to make decisions while their voice still counts. Biomarkers and treatments matter only insofar as they support those goals.
Navigating this territory requires holding complexity — understanding what's within your control, what isn't, and how to plan wisely for both. It means neither over-relying on prevention promises nor surrendering to fatalism. It means understanding that the science is advancing rapidly, that what we can measure and treat today was impossible five years ago, and that thoughtful engagement beats both wishful thinking and despair.
This is part of what I help families do: make sense of the science as it actually stands, understand what it means for their specific situation, and build a path forward that accounts for both what we know and what remains uncertain.
AI Can Help Interpret Your Health Data. That Is Not The Problem That Matters Most.
The new health tools from OpenAI and Anthropic are impressive. They also may not address what matters most for families navigating cognitive change.
OpenAI and Anthropic—two of the biggest names in AI—have launched health products within days of each other. Both let you connect your medical records and get personalized health insights. The various AI chatbots and tools available are impressive, they feel different, they have helped me do and understand things quickly and in new ways, but they have often convinced me of things that were not true, too.
This may remind some of us of the early WebMD and IBM Watson days. WebMD made health information accessible but didn't solve complex health decisions. IBM Watson promised AI-powered clinical intelligence but did not and was never accessible to regular people anyway. Now OpenAI and Anthropic have essentially combined both—AI-powered analysis that anyone can access.
Twenty years ago, WebMD was supposed to transform how we understood our health. And in many ways, it did—millions of people gained access to medical information that was previously locked away in textbooks and doctor's offices. It was a real improvement. It also didn't replace the need for doctors, didn't solve the problem of navigating complex health decisions, and didn't help families figure out what to actually do when facing something serious.
These new AI tools are more sophisticated than WebMD. They can synthesize your personal health data, translate medical jargon, and provide tailored responses. For many health questions, they'll be genuinely useful.
But if you're an adult child watching your parent struggle—wondering whether it's time to have the conversation about driving or finances, trying to coordinate care from a distance while family members disagree about what to do—you may find that these tools, like WebMD before them, don't quite address the problem you're actually facing.
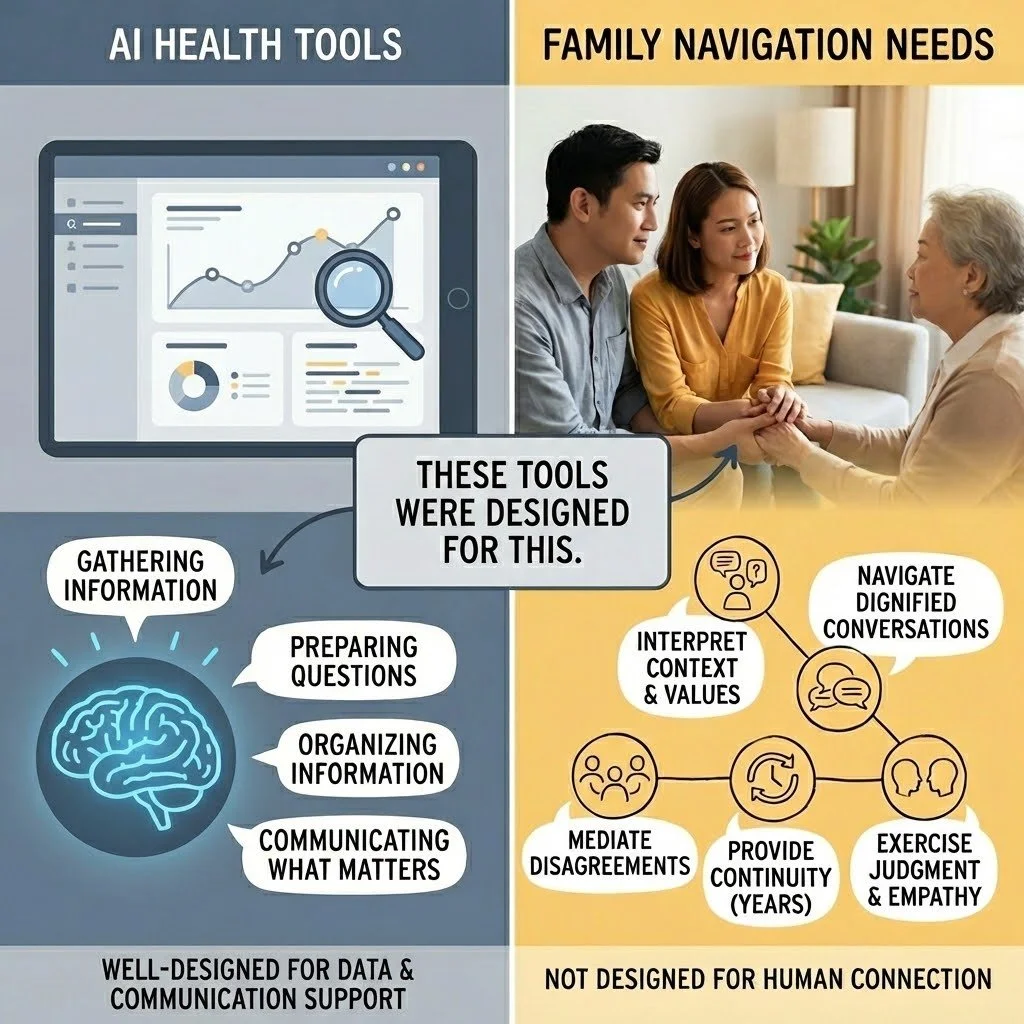
What These Tools Were Designed For
Let's start with what these tools do well, because they do some things very well.
They were designed to help individuals understand their own health data. They pull together lab results from multiple providers. They translate medical terminology into plain language. They can identify patterns across health metrics, test results, and clinical notes. They're available at 2am when you're worried about a test result.
OpenAI reports that 230 million people ask ChatGPT health questions every week. Anthropic's head of life sciences captured the appeal: "When navigating through health systems and health situations, you often have this feeling that you're sort of alone and that you're tying together all this data from all these sources."
That's a real problem, and for many people—particularly those managing their own health with full cognitive capacity—these tools offer a real solution. They were well-designed for that purpose.
The question is whether that purpose matches what families navigating cognitive change actually need.
What These Tools Weren't Designed For
These tools were built on certain assumptions. The user is managing their own health. The user can evaluate whether the information seems accurate. The user can take action on what they learn. The user has access to the relevant health data.
For families navigating cognitive change, those assumptions often don't hold.
The person who needs help may not recognize they need it. Cognitive decline often impairs insight—a phenomenon clinicians call anosognosia. Your father may not notice the changes you're seeing, or he may be frightened and expressing that fear as anger when you raise concerns. AI can synthesize his medical records. It wasn't designed to help you navigate a conversation with someone who doesn't believe anything is wrong. The models weren’t trained on anosognosia data and I have not seen research that shows the models perform well in clinical cases involving social interaction when one partner has limited insight.
You may not have access to the data. These tools assume you're connecting your Apple Health, your patient portal, your lab results. But you're trying to help someone else—someone who hasn't given you access (or not full access), who may not remember their passwords, whose medical information you might legally be unable to see. Medical records often don’t even include the data that matters most for managing care decisions. Can you get an answer? Yes. Is that answer accurate? Unclear. Is that answer helpful? Unclear. Is that answer harmful? Unclear.
Family decisions aren't individual decisions. Your brother thinks you're overreacting. Your sister thinks Mom should be in a facility. You're trying to coordinate care while managing everyone's emotions, your own guilt, and a lifetime of family dynamics. These tools were designed for one person asking about their own health, not for the complexity of family decision-making.
Information wasn't the bottleneck. You've probably already Googled more than you wanted to know about cognitive decline. You've read the Alzheimer's Association materials. You may have a folder of articles about power of attorney and warning signs. The information exists. What you need is help figuring out what it means for your family and what to actually do about it.
What the Data Can't Tell You
AI health tools are only as good as the data they're trained on and the data they can access. Both have significant limitations for families navigating cognitive change.
Training data reflects healthcare's existing patterns. These models learned from existing medical records, research studies, and health information. If the healthcare system has historically under-diagnosed cognitive decline in certain populations, dismissed family concerns, or failed to document early warning signs, AI trained on that data will inherit those blind spots. The models are excellent at pattern recognition—but they can only recognize patterns that exist in their training data.
Medical records capture what gets documented, not what's happening. Your mother's medical record shows her last visit was "unremarkable." It doesn't capture that she's been hiding unpaid bills for six months, that she gave $5,000 to a phone scammer last week, or that she no longer recognizes her grandchildren's names. AI can only access the slice of reality shared with it.
Some populations are systematically underrepresented. A widely-cited study found that a common healthcare algorithm underestimated the health needs of Black patients by using prior healthcare spending as a proxy—encoding historical inequities into automated recommendations. When certain communities are underrepresented in training data, AI tools may work less well for exactly the populations that already face barriers to care.
Rural, older, and less digitally-connected populations may be excluded entirely. Nearly 30% of rural adults lack access to AI-enhanced healthcare tools. Adults over 75 are most likely to struggle with digital interfaces and most concerned about data privacy. The people most likely to be navigating cognitive changes are often least able to benefit from these technologies.
What Families Actually Face
Here's what I've learned about what families are actually navigating:
The conversations are harder than the information. Knowing your father's executive function has declined is one thing. Telling him he shouldn't manage the family finances anymore—without destroying your relationship—is something else entirely. These conversations require timing, judgment, and deep knowledge of family dynamics that no tool can provide.
The medical system often doesn't help until there's a crisis. You've mentioned your concerns to doctors. They did a brief cognitive screen, said "normal for age," and moved on. Meanwhile, you're watching someone make increasingly risky decisions and no one seems to take it seriously. AI tools that connect to medical records from those same dismissive appointments don't change this dynamic. AI tools that don’t have the information that matters most cannot integrate that information into guidance and care.
Caregiving is relentless and invisible. Caregiving is hard. The role demands constant vigilance, difficult decisions, and managing someone who may resent your help. Caregivers describe becoming "like a manager"—deciding everything on behalf of another person while that person often fails to understand why their autonomy is being taken away. This isn't an information problem. It's a human problem.
What you need changes over time. The questions you face in year one are different from year three or year seven. Decisions about healthcare, living arrangements, and finances unfold through ongoing negotiation within families. If the AI tool cannot accurately track those changes over time or treats each question in isolation or without full context, you may not get the guidance and support you need.
Where AI Fits—And Where It Doesn't
AI tools have been incredibly useful in some aspects of my work. They're useful for organizing information, troubleshooting, idea generation and testing, drafting documents, and surfacing patterns across work tasks and items. They help me like a clinical or research assistant I can bother at 2am. I see AI health tools serving a useful purpose in the future if all the risks and concerns are adequately addressed and if people want to use these tools. They may help surface data earlier, prepare better questions for appointments, and reduce some information-gathering burden.
But I see major gaps for what families navigating cognitive change most need:
Interpret data in the context of a specific family's situation, values, and dynamics
Navigate the conversations that preserve dignity while acknowledging hard truths
Mediate disagreements between family members with different perspectives
Provide continuity over a journey that spans years, not queries
Exercise judgment about when to push, when to wait, and what this particular family can actually live with
This isn't a criticism of AI. These tools were well-designed for what they were designed for. They just weren't designed for this.
The Trust Question
Only 5% of Americans say they trust AI "a lot" for health decisions. Trust in AI health information is actually declining—30% now express distrust, up from 23% two years ago. Most people still view their doctor as the most trusted source. It is less clear how people will view their doctors when they partner with AI to provide care.
That skepticism may be healthy. These tools are new. They haven't yet demonstrated reliability in high-stakes, complex family situations. For families facing cognitive decline—where the stakes involve autonomy, dignity, safety, and relationships that span decades—caution seems appropriate.
The question isn't whether AI is good or bad. It's whether these particular tools, designed for these particular purposes, address what families actually need. That families and providers understand the risks and that families and providers know how to use the tools to deliver the most benefit for what matters most to families. This means adequately accounting for biases in the training data, models, and risks that come with turning over aspects of care to a new technology.
This isn't about AI competing with doctors or AI being forced on providers and families. It's about filling a gap that neither fills: helping families interpret what's happening in context, navigate the conversations that matter, and make decisions they can live with over the years this unfolds. The stakes are high to do this in the right way centering the families that are navigating these changes together.
I Want to Play More Golf with Men Who Provide Care
A man I spoke with recently is navigating his wife's early cognitive changes. He's done everything right by the planning playbook—the logistics are solid: finances, decisions, problem-solving. He’s got that. That's the role he knows.
During our conversation, he mentioned a friend who got a care companion so he could play more golf. I have a complicated history with golf, so it is not the place I go for therapy. However, we weren’t talking about golf, we were talking about care. The care for his wife he provides and the support he needs. What I heard underneath: I don't want to escape from this. I want to know how to be in it.
What if he could play more golf with men who provide care?
What the Research Actually Shows
More men are finding themselves in the caregiving role than in prior generations yet most of what we know about supporting caregivers comes from research on women.
When researchers look at male and female dementia caregivers, the findings are more nuanced than you might expect.
Quantitatively, the differences are modest. Across studies, the measurable differences between male and female caregivers are smaller than stereotypes suggest.
Qualitatively, something different emerges. When researchers interview male caregivers, they describe distinct approaches to help-seeking. Men tend to focus on functional tasks first. They're less likely to identify as "a caregiver." They often wait for a kind of permission—from the person with dementia, from a professional—before seeking services. They may not know what the right services look like or who provides what their partner needs. They're patterns shaped by life experience that have real implications for how we design support.
What Male Caregivers Actually Face
Loneliness and isolation. This may be driven by reluctance to leave their loved one alone, friends gradually dropping away, and limited social networks that shrink further during caregiving.
Priority on maintaining couplehood. Male caregivers appear more concerned than female caregivers about being separated from their spouse. They may be reluctant to plan for respite care. Preservation of the relationship itself may take priority.
An unfamiliar role. For men socialized in traditional gender roles, caregiving represents new territory. Men may not see themselves as caregivers, but more importantly, men may not have been socialized to provide care. They may not know how to flex their emotional intelligence in quite this capacity, direction, or in such a sustained manner.
What the Evidence Says Actually Helps
In 2020, the Agency for Healthcare Research and Quality published a comprehensive systematic review of 627 studies on dementia care interventions. In 2021, the National Academies of Sciences, Engineering, and Medicine used this review to develop national recommendations.
The findings were sobering—and clarifying.
Only two types of interventions showed evidence of benefit strong enough to recommend for broad implementation:
Multicomponent caregiver interventions like REACH II (Resources for Enhancing Alzheimer's Caregiver Health), which combines education, skills training, problem-solving strategies, stress management, and support—delivered through a mix of in-home sessions, phone support, and group discussion. Low-strength evidence shows this approach reduces caregiver depression.
Collaborative care models that use multidisciplinary teams to integrate medical and psychosocial care. Low-strength evidence shows these improve quality of life for people living with dementia and reduce emergency room visits.
For all other interventions—including support groups as standalone approaches—the evidence was insufficient to draw conclusions. This doesn't mean they're ineffective. It means we can't yet determine from the research whether they work, for whom, or under what circumstances.
The National Academies report put it plainly: given the complexity of dementia care, mixed results across studies may reflect differences in populations, settings, or how interventions were implemented—not necessarily that the interventions don't work.
Translation: "Just talk about it" is rarely enough on its own. What the evidence supports is a playbook—education, skills, problem-solving, and support—customized to the situation you're actually in.
Bridging the Gap for Men
Here's what we don't yet have: rigorous research testing whether adapting interventions specifically for male caregivers improves outcomes. A 2023 systematic review explicitly noted that none of the digital education studies examined had reported outcomes separately by gender or adapted their approaches for male needs.
That's a significant gap. We're largely working from reasonable inferences rather than proven approaches.
That said, several principles emerge from what we do know:
Start with the practical. Men in caregiving situations often focus on functional issues first. Building rapport may work better when conversations begin with concrete challenges—managing medications, handling repeated questions, navigating the healthcare system—rather than asking about emotional impact directly.
Frame learning as skill development. The evidence shows psychoeducation works. For some men, framing this as building competence in a new domain may resonate better than framing it as processing emotions.
Create space for connection without forcing it. Qualitative research suggests some male caregivers value being around other men in similar situations. But the research does not conclusively show that men-only groups are necessary—only that some men find them valuable.
Address loneliness directly. Given how consistently loneliness appears in the research, any support for male caregivers should explicitly address social connection and isolation.
What This Man Might Actually Need
The man I spoke with doesn't need someone to tell him what to plan. He's done the planning. Based on what the evidence suggests, what might help him is:
Understanding what's changing—translating the science of cognitive change into something he can work with
Concrete strategies—what to say when she asks the same question, how to respond when she doesn't recognize familiar places, when to adjust expectations
A map of what's coming—the predictable decision points he'll face, and when the window to address them starts closing
Permission to struggle—hearing that this is hard, that finding it hard doesn't mean he's failing
Someone in his corner—not for one consultation, but for the journey
References
Butler M, Gaugler JE, Talley KMC, et al. Care Interventions for People Living With Dementia and Their Caregivers. Comparative Effectiveness Review No. 231. AHRQ Publication No. 20-EHC023. Rockville, MD: Agency for Healthcare Research and Quality; August 2020.
National Academies of Sciences, Engineering, and Medicine. Meeting the Challenge of Caring for Persons Living with Dementia and Their Care Partners and Caregivers: A Way Forward. Washington, DC: The National Academies Press; 2021.
Pöysti MM, Laakkonen ML, Strandberg T, et al. Gender differences in dementia spousal caregiving. Int J Alzheimers Dis. 2012;2012:162960.
Sjøvoll TH, et al. "Seeking a Bridge Over Troubled Waters": Older men's experiences as dementia caregivers—A meta-synthesis. BMC Geriatr. 2025.
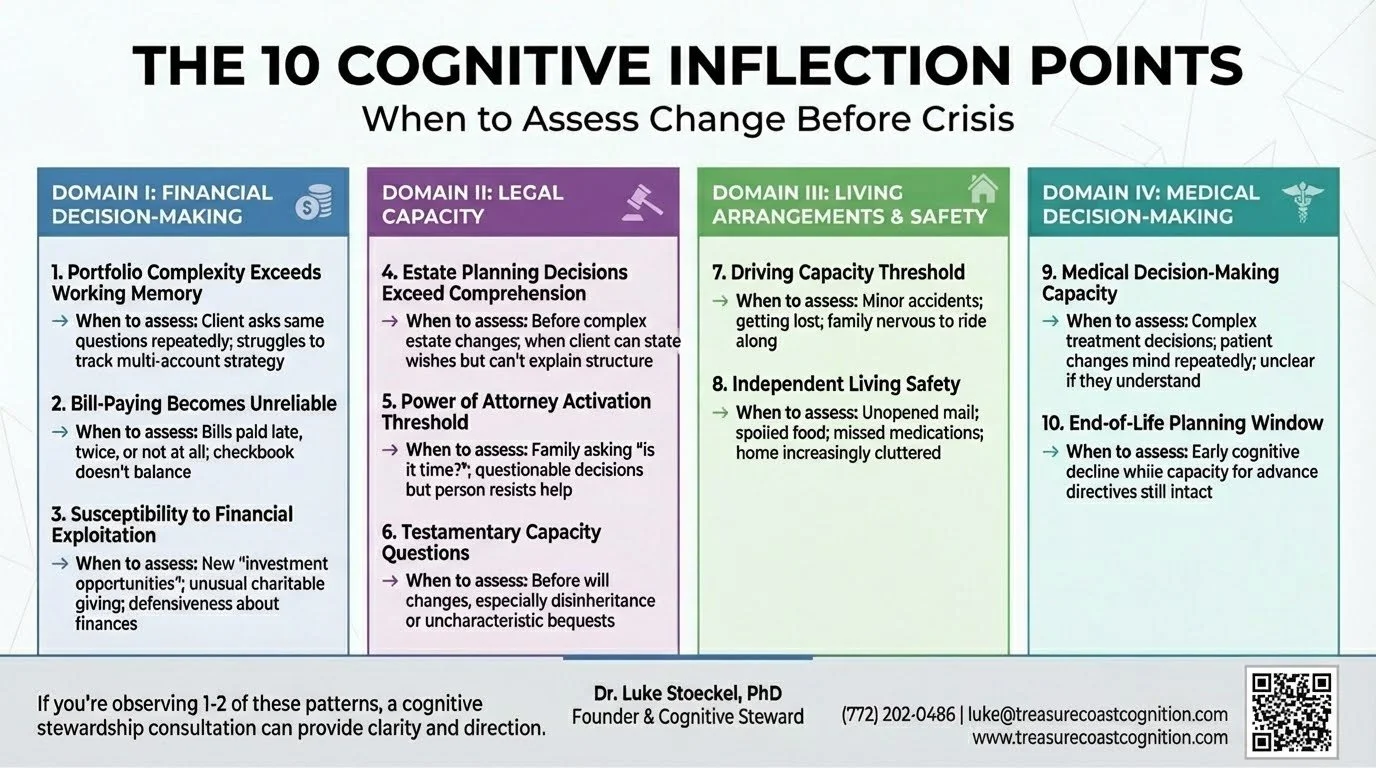
An inflection point is a moment when a relatively small cognitive change creates disproportionate risk for poor outcomes.
These aren't binary thresholds where capacity suddenly disappears. They're gradient transitions where specific cognitive functions decline in ways that impact specific real-world decisions.
The framework identifies 10 predictable decision points that most families encounter during cognitive transitions. If you recognize them early, proactive planning is still possible. If you miss the window, you're managing crisis.
Here's what to watch for:
DOMAIN I: Financial Decision-Making
1. Portfolio Complexity Exceeds Working Memory
What to watch for:
Difficulty tracking multiple accounts
Conversations with advisors require more repetition
Struggling to synthesize overall investment strategy
Increasing reliance on written summaries
The cognitive change: Working memory decline—difficulty holding and manipulating multiple pieces of information simultaneously
Why it matters: The person may still be able to discuss individual investments, but complex portfolio rebalancing, tax strategy, and multi-year planning may exceed current capacity. Without recognition of this change, they may agree to strategies they don't fully understand or resist necessary changes because they can't track the reasoning.
The decision point: Should we simplify the portfolio to match current capacity, or add oversight while maintaining complexity?
2. Bill-Paying Becomes Unreliable
What to watch for:
Bills paid late, twice, or not at all
Checkbook doesn't balance
Confusion about automatic payments
Insistence they paid something they didn't
The cognitive change: Executive function and prospective memory decline—difficulty remembering to perform future tasks and monitoring what's been completed
Why it matters: Bill-paying errors are often among the first visible signs that cognitive changes are affecting instrumental activities of daily living. Late payments can damage credit scores, duplicate payments drain accounts, and unpaid bills create legal consequences. More importantly, if bill-paying is compromised, more complex financial management is almost certainly at risk.
The decision point: Is this occasional forgetfulness that can be addressed with organizational systems, or is this evidence of declining capacity that requires direct oversight?
3. Susceptibility to Financial Exploitation
What to watch for:
Sudden interest in questionable "investment opportunities"
Difficulty ending sales calls
Charitable giving disproportionate to values or means
New "advisors" the family hasn't met
Defensiveness when questioned about financial decisions
The cognitive change: Decline in judgment, emotional regulation, and social cognition—particularly the ability to detect deception, assess risk, and resist persuasion
Why it matters: Elder financial exploitation is estimated to cost older adults billions of dollars annually, with individual losses often averaging $120,000. Once exploitation begins, it tends to escalate. Victims are often too embarrassed to report it, and by the time family members discover it, significant assets may be gone.
The decision point: Can they still manage financial relationships independently, or do they need protective oversight while preserving as much autonomy as possible?
DOMAIN II: Health & Medical Decision-Making
4. Medication Management Becomes Unreliable
What to watch for:
Medications taken at wrong times or in wrong doses
Pills remaining in organizers days after they should have been taken
Confusion about which medication treats which condition
Duplicate or missed doses
The cognitive change: Executive function and prospective memory decline affecting the ability to follow complex medication regimens
Why it matters: Medication non-adherence is associated with increased hospitalizations, disease progression, and preventable complications. For conditions like hypertension, diabetes, or heart disease, inconsistent medication use creates serious health risks. It's also an early indicator that other complex health management tasks may be compromised.
The decision point: Can medication management be maintained with organizational systems and reminders, or is direct oversight required?
5. Complex Medical Decisions Exceed Comprehension
What to watch for:
Struggling to weigh treatment trade-offs
Asking appropriate questions but difficulty retaining information
Changing mind repeatedly about treatment preferences
Physician uncertain whether they truly understand implications
The cognitive change: Decline in complex reasoning, future orientation, and ability to weigh probabilistic outcomes
Why it matters: Medical decision-making capacity is decision-specific and task-dependent. Someone might retain capacity for routine medical decisions but not for complex treatment choices involving risk/benefit analysis and uncertain outcomes. Without proper assessment, patients may make choices they don't fully understand, or family members may inappropriately override autonomous preferences.
The decision point: Can they make this specific medical decision independently, or is supported decision-making needed to ensure understanding?
6. Health Maintenance Tasks Decline
What to watch for:
Missed medical appointments
Laboratory work that doesn't get completed
Recommended screenings or follow-up visits falling through the cracks
Health conditions worsening due to lack of consistent management
The cognitive change: Executive function decline affecting planning, initiation, and follow-through on health maintenance activities
Why it matters: Preventive health care and chronic disease management require consistent execution of multi-step tasks—scheduling appointments, attending visits, following up on recommendations, and coordinating between providers. When executive function declines, these tasks can fail even when the person intellectually "knows" they should do them.
The decision point: Can health maintenance be sustained with reminders and organizational support, or does someone need to take over coordination?
7. Advance Care Planning Window
What to watch for:
Early cognitive decline but still able to discuss future care preferences
The conversation keeps getting postponed because it's difficult
Window for autonomous decision-making gradually closing
The cognitive change: Capacity for advance care planning remains intact, but progressive decline is foreseeable
Why it matters: This represents the last opportunity for the person's own voice to meaningfully guide future medical care. Once capacity is lost, families must make decisions without clear guidance from the person, often leading to conflict, guilt, and choices that may not reflect the individual's values.
The decision point: Is this the appropriate time to document preferences while decision-making capacity is intact?
DOMAIN III: Living Arrangements & Safety
8. Driving Capacity Threshold
What to watch for:
Minor traffic incidents or fender-benders
Getting lost in familiar areas
Family members feeling anxious when riding along
Slower reaction times
Defensive reactions and insistence they're "fine" despite observable concerns
The cognitive change: Possible decline in processing speed, visual-spatial function, divided attention, reaction time, judgment, or executive function—all critical for safe driving
Why it matters: Driving represents independence and identity for most adults. Loss of driving privileges is often one of the most emotionally charged transitions in cognitive aging. Yet continuing to drive with significantly impaired capacity creates safety risks for the driver and others. This requires objective assessment, not family opinion or self-report alone.
The decision point: Can they still drive safely without restrictions? Do they need limitations (daylight only, familiar routes, limited radius)? Must they cease driving?
9. Independent Living Safety
What to watch for:
Unopened mail accumulating
Spoiled food in refrigerator
Missed medications
Home increasingly cluttered or unkempt
Basic home maintenance not occurring
Safety hazards developing
Insistence they're managing fine despite visible evidence to the contrary
The cognitive change: Executive function decline affecting initiation, planning, and organization of daily tasks
Why it matters: This is the inflection point where "aging in place" transitions from preference to potential safety risk. Concerns include fall risk, medication errors, nutritional deficits, fire hazards, and vulnerability to exploitation. However, moving someone from their home prematurely can accelerate cognitive and functional decline and significantly impact quality of life.
The decision point: Can safety be maintained at home with appropriate supports, or has capacity declined to the point where a more structured living environment is necessary?
10. Social Isolation and Support System Erosion
What to watch for:
Decreasing engagement with friends and community
Not returning phone calls
Declining social invitations
Forgetting social commitments
Informal support network no longer in place
The cognitive change: Executive function and social cognition changes that make maintaining relationships more effortful, combined with reduced initiation and follow-through
Why it matters: Research indicates that social isolation is associated with accelerated cognitive decline, increased vulnerability to exploitation, and loss of the informal safety net that often identifies problems early. Without regular social contact, dangerous situations can develop undetected. Isolation may also increase dependence on whatever limited relationships remain—potentially increasing exploitation risk.
The decision point: Is the current level of social engagement adequate for safety and well-being, or does structured social support need to be introduced?
What to Do With This Framework
For Families
If you're noticing changes and wondering "is this normal aging or something more?"—that awareness is important.
Don't wait for crisis. Don't wait for formal diagnosis.
Consider these questions:
Is your parent managing finances and medications reliably?
Are health decisions becoming difficult to navigate independently?
Is driving safety becoming a concern?
Is independent living creating safety risks?
Is their support system diminishing?
If you're observing difficulties in 1-2 of these areas, it may be appropriate to seek professional assessment.
For Physicians
Routine brief cognitive screening can detect dementia but doesn't assess functional capacity for specific real-world decisions.
When patients are facing major medical or financial decisions, or when you observe functional decline in health management despite normal screening results, consider referral for comprehensive assessment including decision-specific capacity.
For Wealth Advisors
When you observe:
Portfolio management discussions requiring more repetition than previously
Uncharacteristic investment decisions
Difficulty tracking multiple accounts that were previously well-managed
Increased reliance on your recommendations without apparent understanding
Family members expressing concerns
Consider: Does my client's current cognitive capacity match the complexity of their portfolio and financial decision-making requirements?
From Reactive to Proactive
The traditional approach to cognitive aging is reactive: wait for crisis or formal diagnosis, then respond.
The 10 Cognitive Inflection Points framework enables proactive stewardship: identify specific decision points when comprehensive assessment should inform planning, intervene before crisis occurs, and navigate transitions strategically while preserving autonomy.
This framework serves:
Families - by transforming vague worry into specific, observable indicators and actionable steps
Physicians - by connecting brief cognitive screening to functional decision-making capacity assessment
Wealth advisors - by matching portfolio complexity to client cognitive capacity and identifying when oversight may be needed
The individuals themselves - by preserving autonomy where capacity exists while providing appropriate protection where capacity is declining
What Cognitive Stewardship Provides
Think of it as proactive planning for cognitive health, analogous to how you might work with a financial advisor for wealth management.
Just as you wouldn't wait until a financial crisis to plan for retirement, proactive planning for cognitive changes can prevent crises while preserving quality of life.
Comprehensive Assessment
Understanding current cognitive strengths and areas of change
Identifying which decisions can be made independently and which may need support
Establishing documented baseline for future comparison
Strategic Planning
Creating a roadmap informed by likely trajectory
Preserving independence and autonomy while addressing identified risks
Developing a proactive plan rather than crisis-driven reactions
Ongoing Professional Partnership
Regular monitoring as cognitive capacity evolves
Adjusting interventions and supports before crises develop
Expert guidance through one of life's most challenging transitions
The Bottom Line
Cognitive Stewardship is not about "taking over" or removing autonomy.
It's about providing clear information and strategic guidance so families can make informed decisions together while respecting the individual's remaining capacity and preferences.
If you're observing difficulties in 1-2 of these areas, you may be in the window where proactive planning can still make a meaningful difference.
Ready to learn more?
Schedule a complimentary consultation to discuss what you're noticing and whether comprehensive assessment and cognitive stewardship planning might be appropriate for your family.
Treasure Coast Cognition
Luke Stoeckel, PhD
Licensed Clinical Neuropsychologist
📞 (772) 202-0486
✉️ luke@treasurecoastcognition.com
🌐 www.treasurecoastcognition.com
Serving families in Vero Beach and throughout Florida's Treasure Coast
© 2025 Treasure Coast Cognition LLC. All rights reserved.
Cognitive Care is a Family Affair
"When I Want Your Help, I'll Ask"
Note: Names and identifying details have been changed to protect family privacy.
How one family went from isolated concern to open conversation in four weeks—and what it means for families navigating cognitive aging:
"When I want your help, I'll ask."
The message was clear. The boundary was firm. The door was closed.
This is what an adult child heard from their parent this past fall when they tried—gently, carefully—to raise concerns about their loved one's memory, confusion with bills, the unsafe moments they'd witnessed.
The adult child backed off. What else could they do? These were their parents' private matters. They lived hundreds of miles away. They had no standing, no permission, no right to push.
So they watched. And worried. And waited for things to get worse.
THE IMPOSSIBILITY
If you're reading this, you probably know this feeling.
You see changes, you experience changes. Subtle at first, then less so. Memory lapses. Confusion about dates. Difficulty with tasks that used to be automatic. Concerning decisions about money or safety.
But when you try to raise concerns—when you suggest it might be time to think about next steps and what those next steps might be—you hit a wall.
"I'm fine." "You're overreacting." "Mind your own business." "When I want your help, I'll ask for it."
And the conversation dies. The concern remains. The situation continues.
You're stuck between two impossible choices: stay silent and watch things deteriorate, or push forward and damage the relationship. You don't understand the space between.
So most families do what this family member did. They wait. They watch. They hope for the best while fearing the worst.
Until crisis forces the conversation nobody wanted to have.
WHAT CHANGED
Four weeks later, everything was different.
Not because the loved one's cognition suddenly improved. Not because the spouse changed their mind about privacy. Not because anyone had a miraculous breakthrough in communication skills. There are still important conversations that need to happen where family may still not be aligned.
What changed was this: the family engaged in Cognitive Stewardship.
This is what Cognitive Stewardship meant to this family:
"It was so challenging earlier this year to know that my parents were struggling but to not have permission to engage, support, envision, and create a different trajectory."
"I believe that some of the most connected and fulfilling years of my parents' lives could be ahead, if we can help them see and create that future."
"Prior to the engagement with you, I did not feel that I had permission from my parents to engage in discussions about care, approach to managing care, and healthcare and other providers. These were my parents' private matters."
"The engagement with you has enabled us to talk about and has encouraged more normalization of this need for help, and I think my parents will be more likely to ask for that help in the future."
From "when I want your help, I'll ask" to productive family dialogue.
In four weeks.
WHAT MADE THE DIFFERENCE
It wasn't magic. It was structure.
What we built together didn't come from brilliant insight into family dynamics or superior communication techniques. What we built together started with something simpler and more powerful: permission structure.
When cognitive changes are subtle, families lose the framework for productive engagement. Adult children don't have permission to raise concerns without seeming presumptuous. Spouses don't have permission to suggest help without appearing disloyal. Everyone walks on eggshells around conversations that desperately need to happen.
An expert third party changes this dynamic. Expertise, trust, and partnership with someone outside the family matters.
When there's a structured framework for family meetings, decision guidance, and ongoing monitoring, it normalizes asking for help instead of making it feel like defeat.
Suddenly:
Concerns can be raised without damaging relationships
Data replaces guesswork
The conversation becomes collaborative, not confrontational
Everyone has permission to engage
The same family member who was told to back off described our work this way:
"Throughout the engagement, I've really appreciated your willingness to meet my family where we are and help us take better next steps... You've listened deeply throughout the engagement in ways that are unlike my experience with other healthcare providers and models."
"This enabled me to learn by engaging with you and your feedback—leaving us in a fundamentally different place than where we were when we began our engagement together just four weeks ago."
WHAT "FUNDAMENTALLY DIFFERENT" LOOKS LIKE
Let me be specific about what changed for this family:
Before our engagement:
Adult child shut out of care discussions
Spouse managing alone, overwhelmed but not asking for help
Loved one’s cognitive changes dismissed as "normal aging" or “dementia”
Family members uncertain how to talk about what was happening
Important conversations simply not happening
After four weeks:
Family aligned on loved one's cognitive trajectory and what it means
Clear plan for next steps across medical, legal, and care domains
Spouse normalized asking for support instead of doing everything alone
Conversations about care happening with dignity and respect for everyone
Adult child positioned to help in ways that support rather than threaten
Loved one engaged in the process and thankful to have partnership and support
Difficult conversations that lead to meaningful actions moving forward
The family described the work as having "the potential to be transformative for my loved one and for my family—which means it's priceless."
Their words.
THE 7-10 YEAR WINDOW
Here's what most families don't realize: there's typically a 7-10 year window between the first subtle cognitive changes and a formal dementia diagnosis.
During these years, families face their most consequential decisions:
Should we downsize or relocate?
Who should manage finances?
When should we update estate planning?
Is it safe to drive?
What care arrangements make sense?
These decisions profoundly impact autonomy, safety, finances, and family relationships.
And traditional healthcare is not set up to provide support during this critical window.
Primary care can offer brief screening, but not always. Often: "everything looks normal for your age." Neurology is reactive: you see them after symptoms are obvious. Memory clinics come too late: after diagnosis, when options have narrowed.
So families muddle through alone, making high-stakes decisions without expert guidance, often getting the timing wrong in ways that cost autonomy, money, and family harmony.
This is exactly the gap Cognitive Stewardship fills.
A DIFFERENT APPROACH
Traditional healthcare asks: "Is there a disease we need to diagnose and treat?"
Cognitive Stewardship asks: "How do we help this family navigate this transition with clarity, dignity, and the best possible outcomes?"
It's not medical diagnosis. It's not psychotherapy. It's not crisis management.
It's expert partnership during the years that matter most—when decisions can still be made proactively, when autonomy can still be preserved, when family relationships can still be protected.
Here's what it looks like in practice:
Comprehensive baseline evaluation (not 10-minute screening): 3-4 hours of neuropsychological assessment that provides real data about cognitive trajectory, not just "seems fine for your age"
Longitudinal monitoring (not episodic visits): check-ins that track changes over time and catch concerns early
Decision guidance (not "wait and see"): expert perspective on the cognitive implications of major life decisions as they arise
Family facilitation (not individual patient focus): structured meetings that help families align, communicate, and move forward together
Coordination with your team (not isolated care): integration with attorneys, financial advisors, and medical providers who need cognitive perspective to do their jobs well
WHAT FAMILIES SAY
The family members I've been quoting described their experience this way:
"Your proactive and comprehensive approach to managing cognitive change provided my family and me with an alternative model for engaging with cognitive changes—one that did not accept the changes as immutable, but rather sought to positively influence trajectory."
"I believe this will have a significant impact on health outcomes, as well as my parents' feelings of empowerment and being in a greater position of control regarding their future lives."
The loved one shared: "he has never met a doctor that was so knowledgeable and yet put him very much at ease."
The spouse and primary care partner? The one who said "when I want your help, I'll ask?" They said: "You struck just the right chord, and I was so thrilled to see laughter and joking with you. It has been quite a long time since I've seen this, so thank you! I especially value that you advise us to focus on what can still be done, and that really matters to me. Thank you for helping us through this confusing and challenging time of our lives."
"[This work has] the potential to be transformative for my loved one and for my family—which means it's priceless."
That's the goal. Not to stop cognitive aging. But to positively influence trajectory. To preserve agency. To support families in making decisions on their timeline, not crisis timeline.
To create permission structures that enable the conversations that matter most.
WHO THIS IS FOR
Cognitive Stewardship is designed for families who:
✓ Notice subtle cognitive changes but aren't finding adequate support in traditional healthcare ✓ Face important decisions in the next 6-12 months and need expert guidance on cognitive implications ✓ Want proactive partnership instead of reactive crisis management ✓ Value preserving autonomy and dignity through this transition ✓ Are willing to invest in preventing regret
It's NOT a fit if you need: ✗ Emergency or crisis care (we're proactive and structured) ✗ Medical diagnosis or treatment (we provide guidance and monitoring) ✗ Quick fixes or one-time consultations (we build relationships over time)
A PERSONAL NOTE
I've spent 20+ years studying the exact window this family was navigating—at Harvard, UAB, Massachusetts General Hospital, and then at the National Institutes of Health, most recently, the National Institute on Aging.
My research focused on cognitive and decision-making assessment and intervention, especially early cognitive change detection and decision-making during cognitive transitions. Deep expertise in this precise problem.
And what became crystal clear: The expertise families desperately need during the 7-10 year pre-diagnostic window simply isn't available in most communities.
Which is why I've returned to my hometown of Vero Beach to launch Treasure Coast Cognition.
To provide the specialized support families are looking for and not finding.
To help families move from "when I want your help, I'll ask for it" to "we're navigating this together."
To create permission structures that make impossible conversations possible.
IF THIS RESONATES
If your family is stuck or you know a stuck family—seeing concerning changes but lacking permission to engage, wanting to help but meeting resistance, knowing conversations need to happen but finding doors closed—I understand.
I know what makes the difference between staying stuck and moving forward together.
And I'm here to help.
Whether you're an adult in your 50s-70s noticing changes in yourself, an adult child managing aging parents from across the country, a local family facing difficult decisions together, or a concerned friend—Cognitive Stewardship might be exactly what you need.
Not to fix everything. But to create the framework for moving forward with clarity, dignity, and expert guidance.
The door that felt closed can open. The conversation that felt impossible can happen. The family that felt stuck can move.
I've seen it. Four weeks ago, in fact.
Ready to explore whether Cognitive Stewardship is right for you or a family you know?
The Gift of Early Action
This week, I received an email that told the story of why Treasure Coast Cognition has a role in the community.
Note: Names and identifying details have been changed to protect family privacy.
A local husband wrote to share his wife's Alzheimer's journey. The email came through a chain of community connections. Before I'd even met them, I knew their story because it happened in our community.
There was a diagnosis of Alzheimer's disease (the most common form of dementia). By any measure, they're beyond the Cognitive Stewardship window. The family knew that. But as I heard their story, I was struck by something powerful: the choices they made years before diagnosis have shaped everything that came after.
The Window That Changes Everything
Here's what they shared about their timeline:
4-7 years before diagnosis: Small signs missed at the time (typical)
2 years later: Acknowledgement of what was happening - and immediate action
3 years later: Found community living where they could secure future memory care
6 months later: Official diagnosis
That period - roughly 18 months of knowing something was wrong but before formal diagnosis - that's the window. That's when decisions were made that have preserved dignity, peace of mind, and quality of life together.
They chose their living situation. They built their support system. They established their care team. They had conversations while everyone could still fully participate in them.
These weren't medical decisions. They were life decisions. And they made them while they still had the cognitive capacity and emotional bandwidth to make them well.
The Cost of Waiting
The husband wrote something haunting: "In looking back I realized that I should have recognized the signs much earlier." He's kept a diary, reconstructing the timeline. He now sees signs going back earlier, maybe even much earlier. So do others.
This is the pattern I see repeatedly in research and clinical practice: By the time families get a formal diagnosis, they've already lost 5-10 years of the window when proactive planning could have made the biggest difference.
Those aren't just lost years for intervention. They're lost years for choice.
The husband shared another story: Last January, they noticed a longtime friend repeating stories. He talked with the friend's wife, shared what they'd learned. The friend acted early and has started a new disease-modifying therapy. He "recognizes that they are now on a similar journey."
That conversation - one friend helping another recognize what's happening early enough to act - that's the intervention that matters most. Not a medication. Not a test. Human connection in the service of preserving choice.
Preserve Choice
When I first started thinking about Treasure Coast Cognition's messaging, a friend mentioned Volvo. Not because I wanted to sell safety features. But because Volvo understood something fundamental: people don't buy safety - they buy the future they want to preserve.
Volvo didn't say "three-point seatbelts and crumple zones." They said "they lived." They showed families intact and futures and choices preserved.
That's what Cognitive Stewardship is really about.
This family made choices early that are still paying dividends today:
They still live in their condo, not institutional memory care
They travel internationally with friends (who understand and support them)
They participate in community activities and engage socially
They spend nights in their community living environment by choice, not necessity
Everyone was able to participate in planning this future
These choices exist because they acted early. The wife driving to the appointment where they made the community living deposit. The wife participating in care decisions. While "reluctant" was still possible - before cognitive decline made even reluctance impossible.
We Need Each Other
Here's what moved me most about the husband’s email. After explaining why they're beyond my Founding Families program window, he wrote:
"It may be that the greatest value in any relationship might be in my/our ability to share with others in your program who may be just beginning their journey."
This is what community means in the context of cognitive aging. We need families who are further along to light the path for families just starting out. We need the courage of people who've navigated these transitions well to give permission to people who are afraid to begin.
Clinical expertise matters. Neuropsychological testing matters. Evidence-based interventions matter.
But so does this: A husband who's kept a diary so he can answer doctors' questions accurately. A wife who voluntarily stopped driving and sold her car - no dramatic intervention needed - because the relationship and trust were strong enough. A friend who noticed repeating stories and cared enough to have a hard conversation.
The dementia journey isn't something we do to people or for people. It's something we walk alongside people through. And we walk it together, as a community.
The Holiday Gift We Can All Give
As we move into the new year, here's what’s on my wish list:
If you're noticing changes in yourself or someone you love - memory slips that seem different than normal aging, difficulty with tasks that used to be automatic, subtle shifts in judgment or personality - don't wait for a crisis to force action.
You have a window. It's open right now. The choices you make in this window will shape everything that comes after.
If you've walked this journey and navigated it well - like this family - your wisdom matters. The families just starting out need to know it's possible to do this with grace, with dignity, with continued joy. They need your permission to act early, to have hard conversations, to prioritize choice while choice is still possible.
If you're connected to families in either situation - make the introduction. Build the bridge. We find our way through this together or not at all.
Looking Ahead
I'm planning to meet with the family in January. Not as a clinical evaluation - they have excellent care already. But as a conversation about how families who've done this well might support families just beginning.
I am thankful for the email that landed in my inbox this week, sent by a husband I've never met, forwarded through a chain of community connections, written with candor and courage about the hardest journey a family can walk - that email is exactly why this work matters.
We preserve choice by acting early. We act early by supporting each other. We support each other by building community that doesn't look away from hard things.
That's the gift we can give each other this holiday season and into the new year.
We're all in this together. And we all need each other. 🎁
What is Direct Cognitive Care?
A New Model for Families Navigating Cognitive Changes
If you or someone you love is experiencing early memory changes, you've likely discovered a frustrating gap in our healthcare system. Traditional neuropsychology offers brief evaluations that result in a report and discharge. Insurance-based neurology provides 15-minute medication checks. Neither offers what families actually need: ongoing partnership, comprehensive assessment, and proactive guidance during the years when planning still matters most.
This is why we created Treasure Coast Cognition as a Direct Cognitive Care practice - applying the proven principles of Direct Primary Care and Direct Specialty Care to neuropsychology and cognitive health services.
Understanding Direct Care
Direct Care is a growing healthcare movement that removes insurance companies from the relationship between patients and providers. Instead of billing insurance for each service, Direct Care practices operate on a membership model - patients pay a periodic fee (monthly, quarterly, or annually) directly to their provider in exchange for comprehensive, personalized care.
The Direct Primary Care (DPC) movement started about a decade ago when family doctors and internists recognized that insurance constraints prevented them from actually caring for their patients. By eliminating insurance billing, these physicians could spend 30-60 minutes per appointment instead of 7-12 minutes, offer same-day or next-day access, and communicate directly with patients via phone, email, or text.
The results were transformative: happier doctors, healthier patients, and lower overall healthcare costs.
More recently, medical specialists have begun adopting this same model, creating what's called Direct Specialty Care (DSC). Rheumatologists, cardiologists, neurologists, psychiatrists, and other specialists are discovering that insurance-free practice allows them to deliver the kind of care they were trained to provide.
Direct Cognitive Care brings this proven model to cognitive health.
Why Cognitive Health Needs Direct Care
Consider what happens when someone starts experiencing cognitive changes in the current system:
The Traditional Path:
Family notices memory problems
Primary care doctor does a brief screening (5-10 minutes)
Referral to neurologist (wait time: 3-6 months)
Neurologist appointment (15-20 minutes, focused on ruling out treatable causes)
Maybe a referral to neuropsychology (wait time: 2-4 months)
One comprehensive evaluation (if insurance approves)
Written report sent to referring doctor
Patient discharged back to primary care
Family left wondering: "Now what?"
What's missing: The ongoing partnership families need during the 7-10 year window between first symptoms and formal diagnosis - the exact time when proactive planning, financial capacity assessment, family education, and care coordination could make the biggest difference.
Insurance doesn't reimburse for these services. Traditional neuropsychology practices can't survive providing them. So families navigate this critical period largely on their own, often making decisions without proper cognitive guidance.
Enter Cognitive Stewardship
Direct Cognitive Care removes insurance barriers and makes true Cognitive Stewardship possible. Here's what Cognitive Stewardship looks like in practice:
Comprehensive Assessment
Instead of rushing through a 90-minute evaluation to satisfy insurance requirements, we spend 4-6 hours across multiple sessions getting to know you and understanding you. We use established neuropsychological measures (not proprietary screens), conduct detailed interviews with family members, and assess real-world functioning including financial capacity - a critical but often overlooked indicator of early decline.
Ongoing Monitoring
Cognition doesn't change once and stay stable. Cognitive Stewardship includes monitoring sessions where we track changes over time, adjust recommendations, and address emerging concerns before they become crises. We catch problems early - when intervention still works.
Direct Access
Questions don't wait for scheduled appointments. With Cognitive Stewardship, you have direct access via phone and email between sessions. Worried about a medication side effect? Confused about what to tell the estate attorney? Concerned about a recent incident? You don't wait three months to ask - you reach out, and we respond.
Care Coordination
Your cognitive health doesn't exist in isolation. We communicate directly with your physicians, coordinate with your estate planning attorney, consult with your financial advisor, and speak with adult children or other family members (with your permission). This coordination rarely happens in traditional practice because insurance doesn't pay for it. In Cognitive Stewardship, it's built in.
Family Education and Support
Understanding what's happening is half the battle. We invest significant time educating families about cognitive changes, what to expect, how to plan, and what interventions might help. We help you make sense of confusing medical information and translate research into practical guidance.
Who Cognitive Stewardship Serves
Cognitive Stewardship is designed for families who:
Are experiencing early cognitive changes but not yet diagnosed
Want comprehensive assessment, not just a diagnosis
Need ongoing guidance as cognition evolves
Are making important financial, legal, or healthcare decisions
Value proactive planning over crisis management
Can invest in preventing cognitive decline complications
Want a genuine partnership, not episodic care
This model works best for families in that critical 7-10 year window between first symptoms and formal dementia diagnosis - when stakes are highest, questions are most pressing, and traditional healthcare offers the least support.
What Cognitive Stewardship Is Not
It's important to understand what we're not:
Not concierge medicine. Concierge practices charge fees for access while still billing insurance for services. We provide all services with transparent pricing and no insurance billing at all.
Not a replacement for medical care. We don't prescribe medications or manage medical conditions. We work alongside your physicians, providing the cognitive assessment and care coordination they don't have time for in their insurance-based practices.
Not one-size-fits-all. Every family's situation is different. Cognitive Stewardship gives us the flexibility to customize services to your actual needs rather than fitting you into insurance's predetermined boxes.
The Broader Movement
Cognitive Stewardship is a new category aligned with the Direct Cognitive Care model. This is part of a larger transformation in American healthcare. Thousands of clinicians across all specialties are rejecting insurance-driven medicine in favor of direct patient relationships. They're proving that when providers can focus on patient needs instead of billing codes, everyone benefits: better outcomes, lower costs, less burnout, stronger relationships.
We're bringing this proven model to cognitive health because families facing cognitive changes deserve the same benefits: comprehensive assessment, ongoing support, care coordination, and genuine partnership throughout the journey.
Is Cognitive Stewardship Right for You?
If you're reading this because someone you love is experiencing cognitive changes, ask yourself:
Do you want more than a one-time evaluation and report?
Would ongoing monitoring and guidance help your family make better decisions?
Do you value having an expert partner throughout this journey?
Can you invest in preventing crises rather than just reacting to them?
If you answered yes, Cognitive Stewardship might be exactly what you need.
Traditional healthcare wasn't designed for the kind of comprehensive, longitudinal cognitive care families facing early decline require. Insurance won't pay for it. Hospital systems can't provide it. But Cognitive Stewardship makes it possible.
That's why we built Treasure Coast Cognition as a Direct Cognitive Care practice, offering a new service, Cognitive Stewardship, to meet the needs of families facing cognitive changes, who deserve better than what the traditional system offers.
Resources:
Does Your Smartphone Need Cognitive Support Mode?
We live in a world where an iPhone has 47 different settings we can customize. Airplane mode. Dark mode for evening reading. Focus modes that silence work emails after 6 PM. Screen time limits. A bedtime routine that dims the display and mutes notifications. Even a driving mode that blocks texts while the car is moving.
But there's no setting for the most predictable change of all: cognitive decline.
When loved ones start showing early signs of memory problems—missed bill payments, confusion about unfamiliar emails, susceptibility to urgent phone calls from "the IRS"—what do we do? Most of us improvise. If you see these changes in a parent, you might enable parental controls meant for children. If you have children, you have also struggled to do this for your children, too. Maybe you change passwords and stored them…somewhere. Hope YouTube does it for you? Set up transaction alerts. Create a patchwork of protections that feel simultaneously inadequate, confusing, and ill-fit: how do you know these protections will “work” for your loved one or yourself?
The technology exists. It just wasn't designed for what matters most.
The 7-Year Vulnerability Window
Here's what research tells us: financial and digital vulnerability doesn't emerge suddenly at dementia diagnosis. It begins years earlier—often 5-7 years earlier—in a window when people still have insight and could benefit from graduated support rather than wholesale loss of independence.
Studies tracking tens of thousands of older adults show that financial problems—missed payments, unusual transactions—emerge an average of six years before formal diagnosis. By the time someone is diagnosed with dementia, significant damage may already have occurred.
Adults over 60 lose an estimated $61 billion annually to financial exploitation. Investment scams alone accounted for $538 million in reported losses in 2024. And with AI making voice cloning and deepfakes trivial, the environment is getting exponentially harder.
AI Acceleration
In 2024, a grandmother in Arizona received a frantic call from her grandson. He'd been in a car accident. He needed $15,000 wired immediately for bail. He was crying. She could hear his voice breaking with fear.
She wired the money within an hour.
Her grandson had never been in an accident. The voice was generated by AI from a 30-second TikTok video he'd posted months earlier. Deepfake video technology can create persuasive footage of trusted figures—financial advisors, doctors, family members—saying things they never said.
We're entering an era where the warning signs we taught people to watch for—"does this email have typos?" "does the caller sound like your real grandson?"—no longer protect anyone. The exploitation techniques are evolving faster than human judgment can calibrate, even for people with intact cognition.
For someone with mild cognitive impairment? For someone whose executive function is quietly declining, whose ability to update beliefs in light of new information is compromised, whose processing speed can't keep pace with rapid-fire persuasion tactics? The current digital environment is a targeting system optimized to exploit exactly their vulnerabilities.
The same AI that makes exploitation easier could also make protection possible for the first time. Machine learning already powers sophisticated fraud detection at financial institutions. Behavioral anomaly detection can identify unusual spending patterns, out-of-character transactions, merchant categories associated with scams. The technical capability exists to build protective scaffolding around cognitively vulnerable adults.
But the scaffolding doesn't exist as consumer-facing technology. Not in any coordinated, accessible, appropriately-calibrated way.
We Need AI For Our AI
If you search for solutions today, here's what you might find:
Apple's Assistive Access creates a completely simplified iPhone interface with large icons, minimal apps, and controlled entry/exit via passcode. It's thoughtfully designed—for severe cognitive disabilities. For someone with MCI who still manages most of their life independently but needs guardrails around high-risk decisions, it's far too restrictive. It's a binary switch when what's needed is a dimmer.
Third-party simplified launchers for Android phones—BIG Launcher, BaldPhone—reduce visual complexity and increase touch target sizes. Helpful for navigation, useless for preventing wire transfers to scammers.
Financial monitoring services like EverSafe and Carefull use AI to detect unusual transactions and alert designated family members. They're valuable tools. They're also boutique services that require families to know they exist, navigate signup, pay monthly fees, and integrate them into a care plan. Only a small fraction of at-risk families ever find them. Most discover them after exploitation has already occurred, if at all.
FINRA Rule 2165 permits financial institutions to place temporary holds on disbursements when they suspect exploitation. Rule 4512 requires firms to make reasonable efforts to obtain a Trusted Contact Person who can be notified when there are concerns. These are meaningful regulatory protections. Most families have never heard of these rules. Most financial advisors don't proactively implement them until a crisis forces the conversation.
There’s no standardized smartphone-based “Cognitive Support Mode” or tool that automatically:
detects early cognitive changes,
intervenes to prevent financial or digital exploitation,
and adapts interfaces based on cognitive ability.
What "Cognitive Support Mode" Could Be
First, smartphones do not appear to cause dementia. In fact, engaging with digital tools may even help maintain cognitive function. The problem isn’t technology use — it’s that technology does not adapt as cognition changes. Imagine if it did. When someone received an MCI diagnosis, their neurologist could say: "One thing we know is that financial and digital vulnerability tends to emerge in this stage. There are protections we can enable now. Let's talk about Cognitive Support Mode for you."
Not a single on/off switch. A graduated system that maps to the trajectory of cognitive decline, configured collaboratively with clinical input, adjustable as needs change.
Tier 1: Enhanced Awareness (MCI with intact insight)
The person retains decision-making capacity but could benefit from prompts and friction at decision points:
Confirmations for high-risk actions: "You're about to wire $5,000 to an account you haven't used before. Call [Trusted Contact] to confirm this is legitimate."
Scam detection overlays: When an email exhibits phishing characteristics—unfamiliar sender, urgent language, request for credentials—a banner appears: "This message has patterns commonly seen in fraud attempts."
Transaction summaries: Weekly digest to both the person and a designated family member showing spending patterns, new payees, unusual merchants.
Cooling-off periods: For large purchases or irreversible transactions, a 24-hour delay with easy cancellation option.
The principle: Informational, not restrictive. The person remains in control but gets decision support at critical moments.
Tier 2: Graduated Restrictions (MCI progressing, partial insight)
As capacity declines, more structural guardrails become appropriate:
Merchant category blocks: Disable purchases of gift cards (the #1 scam payment method), block cryptocurrency platforms, restrict wire transfers.
Dual-approval requirements: Transactions over a configured threshold require confirmation from a designated person before processing.
Simplified interface options: Reduce app access to essentials, increase font sizes and contrast, eliminate notification overload.
Velocity limits: Flag rapid sequences of transactions or interactions with unfamiliar contacts as requiring review.
The principle: Preserve autonomy where safe, add guardrails where risky. We want to make routine decisions independently, but high-stakes actions could be protected.
Tier 3: Supported Decision-Making (early dementia, fluctuating capacity)
When capacity is significantly compromised but the person still engages with technology:
Trusted helper mode: A designated trusted helper receives real-time notifications of high-stakes activity and can intervene before decisions are made.
Curated access: Only pre-approved apps and contacts available. New apps or contacts require helper approval.
Read-only sensitive functions: Can view sensitive information, cannot take action.
Voice verification for high-stakes decisions: "To confirm this wire transfer, please call your designated contact at [number]."
The principle: Maintain engagement while preventing exploitation. The person isn't locked out of their digital life, but significant decisions involve support.
Critical Design Elements
For any of this to work—to be ethical, to preserve dignity, to actually protect people—certain design principles are non-negotiable:
Configurability: Not one-size-fits-all. A clinician and family collaborate to set thresholds, restrictions, and notification recipients based on individual assessment. What's appropriate for one person with MCI is patronizing for another.
Transparency: The person knows what protections are in place and why. Not surveillance, but disclosed support. "Transaction alerts are enabled because we agreed this would help catch unusual activity early."
Reversibility: The person can appeal restrictions. Involve the clinician in reassessment. Graduated protections shouldn't become unilateral control.
Privacy-preserving: Alerts about concerning patterns, not keystroke logging. Monitoring without surveillance.
Progressive activation: Start with minimal restrictions, escalate only as needed, de-escalate if capacity improves (medication adjustment, treatment of depression, resolution of delirium).
The technical components exist:
AI behavioral anomaly detection is already deployed for fraud prevention at financial institutions
Apple, Google, and Microsoft already build sophisticated accessibility frameworks
Clinical assessment tools to calibrate appropriate protections exist and are validated (though in healthcare, we sometimes deploy first and validate later)
Why It Doesn't Exist
Here are some common obstacles:
Liability exposure: What if "Cognitive Support Mode" fails to prevent a scam? Lawsuit. What if it's too restrictive and the person loses money because they couldn't execute a time-sensitive transaction? Lawsuit. The safest legal strategy is to do nothing, let families improvise, and disclaim responsibility. Are you disclosing cognitive status by opting in to “Cognitive Support Mode” and are you protected?
Market uncertainty: Who pays for development? Is this a premium accessibility feature? A healthcare intervention covered by Medicare? A family-purchased service? The business model isn't obvious. And there's stigma—will people actually adopt something called "Cognitive Support Mode," or will they avoid it?
Clinical complexity: Cognitive decline isn't binary. It fluctuates. It varies across domains—someone might retain excellent language skills while losing financial judgment. Who certifies someone "needs" Cognitive Support Mode? What's the threshold? How do we handle the person who refuses protections despite clear vulnerability?
Fragmentation: No single entity controls the digital ecosystem. Apple makes the phone OS, but Gmail is Google, banking apps are each institution, shopping happens across dozens of sites. Building comprehensive protection requires coordination across competitors with different incentives and technical architectures.
These aren't trivial problems.
We figured out how to build accessibility features for blind users across competitive platforms. We created standards for wheelchair accessibility in physical environments despite massive coordination challenges. We developed graduated driver's licensing for teens despite liability concerns.
The question isn't whether this is hard, but whether it matters enough to solve.
What Can We Do?
We can include digital safety assessment like Dr. Peter Lichtenberg has done to help prevent scams and fraud and to help those impacted by financial exploitation (Older Adult Nest Egg: https://www.olderadultnestegg.com/). Document not just that someone has MCI, but what specific vulnerabilities exist and what protections are appropriate. Write prescriptions for technological accommodations the same way you'd prescribe physical therapy or occupational therapy. Become comfortable saying, "Based on your assessment, I recommend enabling these protective features."
Don't wait for the perfect solution. Use what's available now—designate Trusted Contacts at financial accounts, enable call blocking on smartphones, set up transaction alerts, consider monitoring services. Seek out clinicians who understand this domain.
The Other Side of AI: Cognitive Support
AI isn't only a threat. The same technology that enables exploitation also opens remarkable possibilities for cognitive engagement and self-discovery: AI as creative partner, memory companion, and tool for maintaining cognitive health.
AI-powered creative expression could enable older adults—including those with MCI—to engage in artistic activities that would otherwise be inaccessible. Generative image tools let people with limited mobility or artistic training create visual art. AI music composition tool could enable musical expression without years of training. Voice-to-text and text-to-speech break down communication barriers. AI companions could engage users in conversations, suggest activities, and remember past interactions. Users interact with these systems 30+ times daily. These would not be replacements for human connection, but they could be bridges during periods of isolation, prompts for engagement, memory aids. Perhaps most intriguingly, AI could help people see their own cognitive and emotional patterns. Speech analysis could detect subtle changes in language complexity, processing speed, emotional tone—changes that might signal early decline but be invisible to the person experiencing them. Rather than waiting for crisis, AI could provide gentle feedback: "You might want to talk to your doctor about these patterns I'm noticing."
Could the same AI that threatens exploitation also enable self-advocacy?
Imagine an AI system that helps someone with early MCI:
- Track their own decision-making patterns over time
- Identify situations where they feel confused or pressured
- Practice recognizing scam scenarios in low-stakes environments
- Maintain creative expression as other abilities decline
- Document their values and preferences while insight remains
The paradox: AI could both enable the scammer who clones a voice and empower the person with MCI who uses AI tools to maintain agency, creativity, and connection longer. I would argue we want something like supportive AI, which operates transparently, in partnership, enhancing remaining abilities rather than exploiting declining ones.
The conversation about "Cognitive Support Mode" isn't just about blocking bad actors. It's about building infrastructure that enables the beneficial uses of AI while protecting against the harmful ones. The technology is neutral. The design choices matter.
A Role for Cognitive Stewardship
Here's the uncomfortable truth: even if every tech company committed tomorrow to building Cognitive Support Mode and we figured out how to protect people who use it, it would be 2-3 years before coordinated solutions reached consumers. Product development, standards negotiations, and clinical validation takes time.
Families facing this now don't have 2-3 years to wait.
We can conduct comprehensive digital safety assessments that go beyond generic cognitive screening to identify specific exploitation risks. It means helping families implement the fragmented protections that exist today—Trusted Contact designation, graduated financial controls, device-level safeguards, monitoring systems—in a coordinated way that preserves autonomy. It means creating decision rules for when to escalate protections and how to have those conversations without destroying trust.
It's the bridge between "research shows vulnerability emerges six years before diagnosis" and "here are the three things you should do this week."
The technology will get better. The evidence base will improve. Coordinated solutions will eventually emerge. But millions of families are in the vulnerability window right now, today, improvising their way through a landscape that wasn't designed for them.
They need guides who understand both the cognitive neuroscience and the practical tools. Who can assess where someone is on the trajectory and calibrate appropriate protections. Who can translate clinical expertise into actionable family plans.
The Question We Face
Cognitive Support Mode doesn't exist, but it can. We could build it.
But even when we do, we'll still need the human expertise to calibrate it appropriately, to implement it ethically, to balance protection with dignity.
The future isn't choosing between technology and clinical judgment. It's both.
The question isn't whether Cognitive Support Mode should exist.
The question is: What do we do for the millions of families who can't wait?
Who's Really Coordinating Cognitive Care? It Matters.
Yesterday, I shared my upcoming Treasure Coast Cognition launch with my friend group. There was immediate reaction - and the need was clear. I received a direct message from someone I'll call Sarah:
"I'm going to share this with my friend Rachel - her dad was just recently diagnosed. We were just having a conversation about this. Amazing work you are doing."
Sarah's message isn't just a nice comment. It's a data point in a pattern I've been observing since this project has been in development - a pattern that has profound implications for how we think about cognitive health services.
The Pattern in the Data
I've observed a striking pattern in engagement:
Approximately 70% of my social media responses come from women
Every consultation inquiry has been initiated by a woman
The comments consistently say "I wish this existed when my mom/dad was going through this"
The direct messages come from daughters coordinating for parents, wives coordinating for husbands, women leading initiatives in the community
This isn't just my anecdotal observation. It aligns with extensive research showing that 60-70% of family caregivers for people with dementia are women. It is often women who make healthcare decisions when families face cognitive concerns.
The Reality of Family Dynamics
Even when the patient experiencing cognitive changes is male, women are disproportionately the ones who:
Notice the changes first. Subtle shifts in memory, judgment, or personality don't always announce themselves dramatically. They emerge in everyday interactions - forgotten appointments, repeated questions, uncharacteristic financial decisions. The person managing the household calendar, coordinating social plans, and maintaining family connections is often the first to notice something's different.
Initiate the conversation. "We should get this checked out" is rarely an easy conversation to start. It requires navigating denial, fear, and sometimes anger from the person experiencing changes. It requires overcoming the "I'm fine" response and the family's collective wish that everything is okay.
Research and vet options. Who's Googling or asking AI about "memory problems" at 11 PM? Who's calling doctor's offices to ask about wait times? Who's reading reviews and asking friends for recommendations? In most families, it's the daughter or wife.
Coordinate the actual care. Scheduling appointments, taking time off work to provide transportation, sitting in waiting rooms, asking the doctor questions, implementing recommendations, managing medications, following up on referrals - this is invisible labor, and it's disproportionately carried by women.
Manage the emotional complexity. The fear, the grief, the uncertainty, the family dynamics, the tough conversations about driving or finances or living arrangements - someone has to hold space for all of this. That someone is usually female.
What the Healthcare System Does Instead
Despite this reality, here's how most cognitive health services operate:
We direct our questions to the person with the concerns, even when the family member brought them to the appointment.
We schedule around the person with the concerns, without recognizing that the coordination burden falls elsewhere.
We measure outcomes in patient-centered terms, without acknowledging the toll on the person doing the coordinating.
We miss the person who's actually doing much of the work.
The Gap This Creates
When a family first notices cognitive changes, they typically face:
Long wait times. Memory clinic appointments often have wait times of 12 months, with some communities facing even longer delays. By the time you get seen, the trajectory has often progressed significantly.
"Just wait and see" responses. Primary care physicians, stretched for time and working within a system that rewards acute care over proactive monitoring, often default to reassurance rather than early intervention.
One-time evaluations with no ongoing guidance. Even when families do get a neuropsychological evaluation, it's typically a snapshot assessment with recommendations but no active monitoring or support through the changes ahead.
The coordination burden falling on whoever has bandwidth. And in most families, that's the daughter who lives closest, the wife who retired first, the adult child who has the most flexible work schedule. Usually women.
The Multi-Year Journey We're Ignoring
Research reveals a predictable but frustrating pattern in the journey from first concerns to formal diagnosis:
First, 2-3 years of family members noticing changes and wondering if they should be concerned. Subtle shifts in memory, repeated questions, uncharacteristic decisions - changes that prompt the question "is this normal aging or something more?" Studies from memory clinics across multiple countries show families typically notice symptoms for 1-3 years before seeking professional assessment.
Then, 6-12 months (maybe longer) waiting for a memory clinic appointment . Even after deciding to seek help, families face substantial wait times. Some communities have waits extending even longer, and primary care physicians often default to "let's wait and see" rather than proactive monitoring.
Then, if Mild Cognitive Impairment (MCI) is diagnosed, it could be an additional 7 years of uncertainty about what comes next. During the MCI stage - which can last 2-7+ years depending on individual factors - families are essentially told to monitor and return for follow-up, with little guidance on what to do during this critical window.
At each stage of this journey, families are making consequential decisions about finances, legal planning, living arrangements, and care - often without expert guidance. The person experiencing changes may still have capacity for important planning conversations, but families don't know how to have them. Early interventions could potentially slow decline, but families don't know what to prioritize.
And throughout this entire multi-year journey, someone is coordinating. Someone is noticing. Someone is researching. Someone is worrying. Someone is managing the family's response.
That someone deserves support.
What It Means to Build Services for the Actual Coordinator
Recognizing who's really coordinating cognitive care has profound implications for how we design services:
Messaging that validates their experience. "You're not overreacting. You're not imagining it. The changes you're seeing are real, and you deserve expert guidance to navigate them."
Services that support the coordinator, not just the patient. This means recognizing that the wife who's compensating for her husband's memory lapses needs strategies for doing so sustainably. The daughter coordinating from three states away needs clear information to make decisions remotely. The adult children navigating sibling disagreements about their parent's care need frameworks for productive conversations.
Proactive rather than reactive care models. Instead of waiting for crisis or diagnosis, offering longitudinal monitoring and guidance during the years when families most need it - when they're noticing changes but the healthcare system says "not yet."
Acknowledging the invisible labor. The coordination work is real work. It takes time, emotional energy, and often comes at the cost of careers, relationships, and personal wellbeing. Services should recognize this reality rather than treating it as incidental to the "real" patient care.
Why Referral Partners Need to Know This
If you're a healthcare provider working with older adults, you've probably seen this dynamic play out:
A couple comes in for medical care. The wife makes the appointment. She's the one asking the questions. She's noticed changes in her husband's thinking and judgment. She wants to know more "just in case."
Or an adult daughter schedules a consultation about her mother's finances. She's noticed confusion about bills, unusual purchases, vulnerability to scams. She's trying to figure out how to join the decision-making process while preserving her mother's dignity and autonomy. This is the shared decision-making threshold.
The person sitting across from you asking these questions? That's your actual client for cognitive health services.
When you're considering referrals for families with cognitive concerns, ask yourself: Who initiated this conversation? Who's been doing the research? Who will be implementing the recommendations?
That's who needs to know about services designed for family coordinators navigating cognitive change.
To the Family Coordinators Reading This
If you're the daughter who took a day off work to bring your father to appointments...
If you're the wife who's been quietly compensating for your husband's memory lapses...
If you're the adult child trying to coordinate care from three states away...
If you're the one who's been Googling "early signs of dementia" and asking AI if you're overreacting...
You're not overreacting. The changes you're noticing are real.
You're not alone in carrying this burden. This is a pattern across millions of families.
And you deserve support that recognizes the work you're actually doing.
The healthcare system wasn't designed for the role you have. But we can redesign the experience to serve the people navigating cognitive uncertainty - not just the patients, but the families, and specifically the women who disproportionately carry the coordination burden.
That's what I'm building with Treasure Coast Cognition's Cognitive Stewardship model: Longitudinal support throughout the multi-year journey from first concerns through diagnosis and beyond, recognizing that families - and particularly family coordinators - need expert guidance to navigate cognitive change with dignity, agency, and clarity.
The question "Who's really coordinating cognitive care?" isn't just an observation about gender dynamics in healthcare (men are caregivers, too).
It's a question we should ask before we design the services that help those coordinating cognitive care.
Your Doctor Said 'Wait and See.' Here's Why That's Wrong.
Something feels different.
Maybe it's you noticing that managing the investment accounts you've handled for decades suddenly feels more complicated. Or it's your spouse forgetting entire conversations you just had. Perhaps it's Mom getting confused about finances she's managed flawlessly for years, or Dad making uncharacteristically impulsive decisions.
You've probably tried mentioning it to the doctor, only to hear: "It's just normal aging" or "Let's wait and see."
But you know something has changed. And you're caught between two impossible choices: ignore the changes and hope things stay stable (while worrying constantly), or take over completely (causing conflict and resentment).
There's a better way.
The 7-10 Year Gap No One Talks About
Here's what most families don't realize: cognitive changes typically unfold slowly over 7-10 years before anyone receives a formal diagnosis. During this long window, families face some of life's most consequential decisions—about finances, legal planning, medical care, living arrangements, and safety—without clear guidance on what's actually happening or what to do about it.
This is the gap where families need help most. And it's exactly when the current healthcare system fails them.
Why the System Leaves You Stranded
When you finally push for evaluation, you might get:
A 5-minute cognitive screening that "looks fine"
A referral to a neurologist with a 6-month wait
Testing only after things have gotten significantly worse
Meanwhile, you're facing real decisions right now:
Should we still manage the investment accounts independently?
Is it safe to drive?
Can we make informed medical decisions?
Should we update the estate documents while capacity is still clear?
How do we address this without destroying our relationship?
What happens if we wait and capacity is lost?
Nobody is helping families during the 7-10 years when help matters most—when cognitive capacity is declining and planning is still possible but increasingly urgent.
Introducing Cognitive Stewardship™
Cognitive Stewardship is expert guidance designed specifically for the years before diagnosis. It's not crisis management after things fall apart. It's not a one-time diagnostic test that tells you "what's wrong." It's not medical treatment, and it's definitely not taking over your loved one's life.
Think of it as a wealth advisor for your brain. Just as you wouldn't wait until bankruptcy to manage finances, you shouldn't wait until crisis to protect cognitive health.
Cognitive Stewardship is:
Understanding exactly how the brain is working right now – not just test scores, but what it means for real life
Knowing which decisions are urgent and which can wait – so you're not constantly reacting
Creating strategies that preserve independence while protecting against harm
Having a plan for the next 5-10 years – a roadmap, not guesswork
Expert partnership through one of life's most difficult transitions – you don't navigate this alone
The 10 Cognitive Inflection Points
Most families face 10 predictable crossroads during cognitive transitions. Each represents a moment when declining capacity intersects with important decisions. If you catch them early, planning is still possible. If you miss the window, you're managing crisis.
These inflection points include:
Financial Oversight (often first) – checkbook errors, vulnerability to scams, poor investment decisions
Driving Safety – represents independence and identity, but safety risks mount
Medication Management – missed doses, confusion about timing, medication errors
Estate Planning (critical window) – last opportunity for autonomous legal decision-making
Housing Transitions – before safety concerns force crisis moves
Healthcare Proxy Activation – when medical decision-making becomes too complex
Advanced Care Planning – expressing end-of-life preferences while capacity remains
Loss of Insight – when the person doesn't realize capacity has changed
24/7 Care Need – transition from intermittent support to full-time care
End-Stage Care – hospice, comfort care, meaningful closure
The key is anticipating these inflection points before you reach them, not reacting after crisis forces your hand.
What Cognitive Stewardship Looks Like
Phase 1: Comprehensive Assessment
We spend 2 days (3-4 hours total) understanding how things are right now. This isn't a quick screening. It's thorough evaluation of cognitive patterns, real-world decision-making capacity (financial, medical, daily functioning, safety), and what matters most to you and the family from your perspective and observations.
This assessment reveals where thinking is still strong (we build on strengths), where things are becoming more effortful (we plan supports), which upcoming decisions require immediate attention, and what the map might look like over the next few years.
Phase 2: Collaborative Discovery Session (90 minutes)
A few days after the assessment, we sit down together—you, your loved one, and me—and explore what we learned. This isn't clinical report delivery. It's a conversation.
We discuss what's working well, what's changing, what this means for real life, and strategies that fit your family. Together, we design practical approaches that preserve autonomy as long as safely possible, protect against specific risks we've identified, feel supportive rather than controlling, and can be adjusted as things evolve.
You leave with clear understanding of what's happening, a roadmap for the next 2-5 years, specific strategies to try, and a plan for ongoing monitoring.
Phase 3: Longitudinal Monitoring (Every 3-12 months)
Cognitive decline isn't a one-time event. It's a journey. What's true today may change in six months.
Regular follow-up sessions allow us to track how things are progressing, see if our strategies are working, identify new concerns as they emerge early, update the roadmap as circumstances evolve, and provide ongoing guidance as you face new decisions.
This ongoing relationship means you're never navigating alone.
What This Investment Prevents
Consider what you're preventing:
Financial exploitation: Average loss per victim: $120,000
Contested estates: Legal fees: $50,000-$200,000+
Crisis placements: Emergency memory care: $8,000-15,000/month
Medication errors: Hospitalizations from medication mismanagement: $15,000+ per incident
Family conflict: What may matter most—objective information reduces disagreement
What You're Gaining
Clarity – Understanding what's actually happening instead of worrying and guessing
Plan – Knowing what decisions are coming and when action is needed
Preservation – Keeping your loved one independent as long as safely possible
Protection – Preventing exploitation, errors, and crisis situations
Peace of mind – Having expert guidance instead of navigating alone
Family harmony – Reducing conflict with objective assessment and shared understanding
Time – Planning during the window when planning is possible, not reacting to crisis
Dignity – Honoring your loved one's autonomy and involving them in decisions about their future
Is Cognitive Stewardship Right for You?
You might benefit if:
You're the person noticing changes:
You're worried about your own memory or thinking
You want to plan ahead while you still can
You want your family to have guidance, not just worry
You have important decisions coming up (financial, medical)
You're the spouse or partner:
Your partner seems different—not just "aging"
You're worried but don't know if you're overreacting
You need someone objective to assess the situation
You're exhausted from constant vigilance and worry
You're the adult child:
You live far away and worry about what you're not seeing
Your parent insists "everything's fine" but you're concerned
You and your siblings disagree about what to do
You want to help but don't want to take over
Your Next Step
The first step is simple: a complimentary 30-minute consultation where we'll talk about what you're noticing, what concerns you most, what decisions you're facing, and whether Cognitive Stewardship is the right fit.
No pressure. No sales pitch. Just honest conversation about whether I can help.
You don't have to navigate this alone. That's the entire point.
The gap between noticing changes and getting a formal diagnosis doesn't have to be filled with anxiety and guesswork. It can be a time of thoughtful planning, preserved dignity, and expert guidance.
Let's figure it out together.
Ready to take the next step?
☕ Schedule Your Complimentary Consultation - Let's discuss your specific situation. No pressure, no sales pitch—just honest conversation about whether I can help.
Schedule 30-Minute Consultation
Thanksgiving Conversations
Thanksgiving: so much turkey, stuffing and canned cranberry jelly; so much time with friends and family; so many important, meaningful conversations. I had three reactions: first, I ate too much; second, people can be incredibly thoughtful, caring, and trusting; and third, we can do better to support how we navigate cognitive health concerns.
Here are some of the questions I heard:
"I live in Georgia, but my Mom lives near Vero. Her husband has Lewy Body Disease and they both need more help, even though her husband may not think so. They don’t have easy access to resources. What can we do?"
"How do we deal with the dysfunction in our local healthcare system?"
[Email]: “We are abroad and my mother-in-law had an unexpected change in medical status. Do we stay for treatment or travel back to the US? What are the flight risks?"
"My p-tau217 is elevated—what does that mean?"
"We’re exhausted. Always. We need support, care, and direction now. What can we do?"
"I’ve been hearing about those weight loss drugs (GLP-1s). Can those help my brain health?"
"Do microplastics disrupt tau clearance? I mean, am I going to hurt my brain if we don’t clean up the Indian River Lagoon?"
These questions all map onto the same three fundamental needs.
1. "What does this signal actually mean? Is this something or is it nothing?"
This is at the heart of what Treasure Coast Cognition does: we tie signal to meaning.
Many people now receive p-tau217 results, Aβ42/40 ratios, inflammatory markers, sleep data from wearables, or confusing research headlines. The challenge is interpretation.
A biomarker is probabilistic, not deterministic. An elevated p-tau217 is a probability, not a diagnosis. Effect sizes in research don't always translate to clinically meaningful change. And what's clinically meaningful isn't always the same as the real-world changes that matter most to you.
The GLP-1 question illustrates this perfectly. The recent Alzheimer's trial results were disappointing—but that doesn't mean GLP-1s don't matter for brain health. What's true for Alzheimer's disease specifically may not be the same as what's true for midlife metabolic risk modification, late-life vascular protection, or pre-dementia cognitive reserve. A negative trial in one disease at one stage doesn't eliminate the potential for meaningful impact in a different context.
Whether the concern is p-tau217, GLP-1 trial results, microplastics, or an urgent medical decision abroad, people are trying to understand: Is this something? Or is it nothing?
At Treasure Coast Cognition, we help individuals and families determine which signals are meaningful—and which are not.
2. "What should I be monitoring over time?"
This is exactly what Treasure Coast Cognition helps you figure out.
People don't just want data—they want direction.
When someone asks "What should I expect?" or "What should I be looking for?" or "How do I know if something is shifting?"—what they're really asking is: Which patterns actually matter, and how do I track them responsibly?
You want to monitor what is meaningful—so you can know when changes are meaningful.
Questions about local healthcare dysfunction reflect this same need. Continuity and longitudinal monitoring are exactly what fragmented systems fail to provide. When the system is broken, people still need guidance—and they need it from someone who understands both the science and the context of where they live.
Traditional healthcare does not provide this continuity. Treasure Coast Cognition does.
3. "What will matter most in the next 5–10 years?"
This question is why I'm building Treasure Coast Cognition here.
Healthcare systems need to work within the context of the places and systems where we live and function. When there's dysfunction in the local healthcare system, we still need to meet the healthcare needs of the community. When someone faces an urgent decision while traveling abroad, they need clarity fast. When a family in a remote setting needs a care plan, they need guidance that works across distance.
Cognitive stewardship has to be embedded in the environment where people actually live.
Questions about why Treasure Coast Cognition exists here, or how to navigate a broken healthcare system, or whether GLP-1s help cognition all point back to a desire for long-term clarity:
How do I stay independent?
How do I protect function?
How do I keep doing the things that matter to me?
What decisions should I prepare for?
What actions actually change my trajectory?
But there's another layer to this question. The microplastics question points to something bigger: What lives in the environment around us that could be changed outside of us to improve our lives? Individual risk modification matters, but so does community-level action to decrease environmental contributors to poor brain health. Cognitive Stewardship isn't just personal—it's also environmental and systemic.
The goal isn't just early detection. It's early direction.
Where the Questions Converge
Even though Thanksgiving brought questions about biomarkers, metabolism, microplastics, healthcare systems, and urgent medical decisions, the underlying focus was the same:
What does this signal mean?
What should I monitor?
What will matter most over the next decade?
Treasure Coast Cognition exists to help people answer these questions with clarity, calm, and evidence—and to help them stay aligned with the life they want to protect.
Interpretation → insight → action.
That's Cognitive Stewardship.
Changes in Financial Decision-Making Can Be the First Sign of Cognitive Decline
The Brain’s Financial Crisis
A 2024 study from the Federal Reserve Bank of New York is the latest alarm bell that missed credit card and mortgage payments can precede a dementia diagnosis by years.
Researchers analyzed data from 2.4 million older adults between 2000 and 2017, linking Medicare claims with Equifax credit report histories. About 478,000 of these individuals were later diagnosed with Alzheimer's disease or related dementias.
Here's what they found:
Financial problems start years before diagnosis.
Credit scores begin to fall and late payments start showing up more than five years before dementia is clinically recognized. Specifically, credit card delinquency increases were observed five years prior to diagnosis, while mortgage delinquency appeared three years before.
By the point of diagnosis, people were over 34% more likely to miss credit card payments and 17% more likely to miss mortgage payments compared to earlier baseline years.
The authors describe "disease-related inattention and decision difficulties"—missed due dates, confused accounts, duplicated payments. The financial decline mirrors the quarter-by-quarter cognitive decline these individuals are experiencing.
Translation: Cognitive decline can show up early in credit scores and bank statements.
Why Finances Fail First
Financial management depends on cognitive skills—many of which decline early in Alzheimer's disease and related dementias.
1. Executive Function
Planning, sequencing, attention, inhibition, and problem-solving—the capacities required to juggle bills, budgets, and deadlines—often show early decline.
2. Prospective Memory
Remembering to do something in the future—pay the electric bill next Tuesday, transfer funds before the autopay hits.
Prospective memory failures are among the most sensitive early markers of decline.
3. Numeracy
Even simple math—calculating interest, comparing prices, checking balances—relies on parietal and frontal brain networks disrupted by early pathological changes.
4. Vulnerability to Exploitation
Subtle cognitive decline—even before mild cognitive impairment is detectable—can result in impaired financial decision-making and increased vulnerability to scams.
5. Increased Altruism and Generosity
Here's a subtle sign that doesn’t seem alarming on the surface: A person who has always been financially conservative suddenly becomes unusually generous.
Research from USC and Bar-Ilan University found that increased financial altruism—giving away more money to anonymous others in experimental tasks—was associated with poorer performance on cognitive tests sensitive to early Alzheimer's disease, including word learning, story recall, and category fluency.
While deliberate generosity is certainly not a negative trait, research suggests that sudden or increased altruistic behaviors may be associated with declining cognitive function in some contexts.
The mechanism appears related to impaired judgment and reduced ability to assess risk and future consequences. The same frontal-executive systems that help us evaluate financial trade-offs and protect our interests are the ones that show early changes in dementia.
Families can interpret this as "Mom's just being nice" or "Dad's finally learning to enjoy his money."
But when generosity represents a significant change from baseline behavior, it may be a signal of cognitive decline.
What the Brain Reveals
In a cohort of 97 cognitively normal adults, researchers found that thinner entorhinal cortex—a structure affected early in Alzheimer's—was linked to greater self-reported vulnerability to financial exploitation (Fenton et al., Cerebral Cortex, 2024).
It's correlational, not diagnostic—but it hints at a neural marker for early financial risk.
In one of my early studies—MRI Volume of the Medial Frontal Cortex Predicts Financial Capacity in Mild Alzheimer's Disease (Brain Imaging and Behavior, 2013)—we found that atrophy in the medial frontal cortex, a hub for attention, calculation, and decision-making, directly predicted poorer financial capacity.
That work, later highlighted by the NIA, helped me discover and understand this:
Financial decision-making could be an early marker of neurodegeneration.
What We Can Do
1. Detect Early
Monitor real-world financial data—credit scores, bill-payment history, online banking patterns, and changes in giving behavior—just as we monitor blood pressure.
2. Plan Legally and Financially While Capacity Intact
Power of attorney, trusts, wills, financial protections, advance directives—complete them before decline.
3. Evaluate Financial Capacity
Financial capacity screening is important, not just screening for cognition. Review bill-paying habits, credit patterns, changes in charitable giving or financial behavior, and transaction histories to identify early warning signs.
Because the earlier we see decline, the more autonomy and dignity we can preserve.
4. Finances Are Personal, So Are The Data
Accessing financial information is sensitive and requires appropriate privacy protections, explicit consent, and security measures that prevent the very exploitation we're trying to detect.
The Bottom Line
Financial errors often precede cognitive diagnosis by years.
Specific brain regions—like the entorhinal and medial frontal cortex—predict financial decline.
Vulnerability to scams tracks with early Alzheimer's-related brain changes.
Increased financial altruism could be a sign of cognitive changes.
If you notice these changes—in yourself or someone you love—don't ignore them. They could be signals from the brain that it's time to pay attention.
References
Gresenz CR, et al. The Financial Consequences of Undiagnosed Memory Disorders. Federal Reserve Bank of New York Staff Reports. 2024;(1106). doi:10.59576/sr.1106
National Institute on Aging. Difficulty Managing Bills May Signal Early Dementia. Published January 16, 2025. Accessed November 12, 2025.
Stoeckel LE, et al. MRI Volume of the Medial Frontal Cortex Predicts Financial Capacity in Patients with Mild Alzheimer's Disease. Brain Imaging Behav. 2013;7(3):282-292. doi:10.1007/s11682-013-9226-3
Fenton L, et al. Lower Entorhinal Cortex Thickness Is Associated with Greater Financial Exploitation Vulnerability in Cognitively Unimpaired Older Adults. Cereb Cortex. 2024;34(9):bhae360. doi:10.1093/cercor/bhae360
Weissberger GH, et al. Increased Financial Altruism is Associated with Alzheimer's Disease Neurocognitive Profile in Older Adults. J Alzheimers Dis. 2022;88(3):995-1005. doi:10.3233/JAD-220187
National Institute on Aging. Increased Financial Generosity Linked to Lower Cognition and May Be an Early Indicator of Alzheimer's Disease. Published June 2022. Accessed November 12, 2025.
National Institute on Aging. Brain Scans Offer Insights into Loss of Money Skills. Accessed November 12, 2025.
Ho EH, et al. A Scoping Review of Financial Decision-Making Measures in Midlife and Beyond: Results from the ARMCADA Study. Front Psychol. 2025;16:1540508. doi:10.3389/fpsyg.2025.1540508
Why I'm Leaving My Office To Come To You
The most important part of a cognitive evaluation doesn't happen in my office.
It happens in your kitchen. Your bathroom. Your bedroom. The places where you actually live.
Because here's what I've learned after 20+ years of neuropsychological assessments: People can look fine in a clinic and be falling apart at home.
Your father aces the memory test in my office—but at home, there are 14 half-empty water bottles lined up on his dresser because he forgets he already got one.
Your mother seems oriented and alert during our appointment—but when I visit her home, the stove has scorch marks and expired food fills the refrigerator.
If I'm not seeing how you function where you live, I'm missing the whole story.
I was explaining Cognitive Stewardship to a physician friend in Vero over coffee. He listened as I described the model. Then he said: "This needs to be part of a new model for primary care. In-home primary care." I paused. "Tell me more."
Patients cycle through the hospital. Elderly, cognitively impaired, medically complex. Dehydration. UTIs that become delirium. Medication errors. Falls. The family doesn't realize it was this bad. And it’s preventable.
The problem isn't that primary care doctors don't care. It's that they can't see what's happening at home. Fifteen-minute office visits don't capture reality. When families arrive at the hospital, it’s to manage a crisis that's been building for months.
We need to go to you.
I come home, open my browser, and a new JAMA Network Open study appears showing primary care can spot early signs of dementia. This randomized clinical trial, led by Dr. Malaz Boustani and colleagues at Indiana University, tested a simple idea:
Can primary care clinics detect early cognitive decline using digital tools that fit into everyday workflows? Yes.
The study compared three approaches across nine federally qualified health centers in Indianapolis:
Usual care (no routine cognitive screening)
A machine-learning Passive Digital Marker (PDM) that analyzed existing electronic health record data to flag patients at risk
A PDM + patient-reported QDRS (Quick Dementia Rating System) combined approach
After one year, the combined approach led to 31% more new diagnoses of Alzheimer's disease and related dementias (15.4% vs 12.4% in usual care). Clinicians also ordered more appropriate diagnostic workups—brain imaging, labs, neuropsychological testing.
The key finding: Primary care can implement early cognitive screening at scale without disrupting workflow or adding clinician burden—and it helps identify people who would otherwise be missed.
Detection Isn't Prevention
But here's the piece we still haven't solved:
Detecting early cognitive changes is helpful—but it doesn't automatically lead to better outcomes for patients or families.
Finding early drift in memory, thinking, or executive function is only meaningful if we can do something with that information.
The limits of the study make this clear:
It focused on diagnosis, not real-world functioning
It didn't track hospitalizations, readmissions, or ED visits
It didn't address medication complexity, sleep disruption, caregiver strain, or home safety
It didn't connect detection to interventions that stabilize people at home
The Real Problems Happen at Home
People don't decline in the exam room.
They decline at home.
Long before a crisis or hospital visit, subtle destabilization shows up in:
Medication confusion (pills missed, duplicated, or taken incorrectly)
Sleep disruption (up all night, exhausted during day)
Mood and behavior changes (irritability, withdrawal, anxiety)
Executive function slips (bills unpaid, appointments forgotten)
Caregiver overwhelm (spouse exhausted, adult children burning out)
Missed follow-up appointments (patient forgets, family can't transport)
Mobility declines (unstable gait, near-falls, reduced activity)
Unsafe routines or environments (stoves left on, spoiled food, fall risks)
These changes are detectable. But right now, nobody is watching.
Early cognitive drift is rarely the sole cause of a readmission or major decline—it's the signal that bigger problems are coming.
By the time families seek help, the crisis has already arrived:
Dehydration → hospitalization
UTI → delirium → ED visit
Medication error → hypoglycemia → ambulance
Fall → hip fracture → surgery → skilled nursing facility
Caregiver collapse → emergency nursing home placement
Many of these crises are preventable.
What This Study Actually Tells Us
The JAMA article validates something important:
With the right tools, primary care can see the early drift.
The Passive Digital Marker (machine learning analyzing EHR patterns) plus the Quick Dementia Rating System (patient-reported symptoms) together changed clinician behavior—prompting diagnostic workup and earlier diagnosis.
We can detect cognitive changes in primary care.
Now we need to build the systems that respond before the drift becomes a crisis.
From Detection to Stabilization at Home
Imagine combining detection with a different care delivery model:
Early cognitive detection (QDRS + digital markers embedded in EHR)
↓
In-home comprehensive assessment (where patients actually live and function)
↓
Integrated primary care + cognitive monitoring (not fragmented across specialists)
↓
Proactive stabilization:
Medication safety monitoring (are pills being taken correctly?)
Sleep optimization (treating sleep apnea, adjusting sedating medications)
Mobility and fall prevention (PT, home safety evaluation)
Nutrition and hydration (addressing weight loss, dehydration before crisis)
Caregiver support (respite, education, burden monitoring)
Decision-preparedness coaching (legal, financial, advance directives)
↓
Continuous monitoring (monthly home visits, not annual clinic check-ins)
↓
Crisis prevention (hospitalizations, ED visits, nursing home placement)
Prevent hospital readmissions and preserve independence not by waiting for the next clinic visit, but by creating a continuous stability layer around the patient at home.
In-Home Primary Care
Traditional office-based primary care is not set up for cognitive change:
Transportation barriers if driving becomes risky or people refuse to visit the clinic
Clinic performance ≠ home performance: Someone may seem fine in a structured 15-minute clinic visit, but they may look very different at home
Medication management invisible: Pill bottles in clinic don't reveal whether pills are being taken correctly
Reactive, not proactive: Patient presents when crisis occurs; no monitoring between visits
Family excluded: Caregivers can't attend every appointment; clinician doesn't see family dynamics
In-home primary care solves these problems:
Direct observation: See how people actually function in their environment
Medication reality check: Pills scattered on counter, organizer filled incorrectly, expired meds not discarded
Home safety assessment: Loose rugs, clutter, spoiled food, burners on
Caregiver assessment: visible exhaustion, adult daughter on verge of quitting job
Early intervention: Detect problems before crises (dehydration, UTI, medication errors, fall risk)
Continuous relationship: Monthly visits create trust; patients more likely to report problems early
When you combine digital cognitive detection with in-home primary care delivery, you create a powerful prevention model:
Early detection identifies patients at risk
Home-based assessment reveals functional realities invisible in clinic
Integrated medical + cognitive management addresses complexity comprehensively
Continuous monitoring catches destabilization early
Proactive intervention prevents crises
Where Do We Go From Here?
Primary care can detect early cognitive decline.
Now we need to take the next step:
Build the systems that turn detection into prevention.
Extend monitoring from the clinic into the home where risk actually unfolds.
Create continuous stability around patients before crises force reactive management.
That's the work ahead.
My hospitalist friend was right. We need to go to them. We can detect the problems. Now we need to build the systems that prevent the crises—one home visit at a time.
Reference
Boustani MA, Ben Miled Z, Owora AH, et al. Digital Detection of Dementia in Primary Care: A Randomized Clinical Trial. JAMA Netw Open. 2025;8(11):e2542222. doi:10.1001/jamanetworkopen.2025.42222